This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
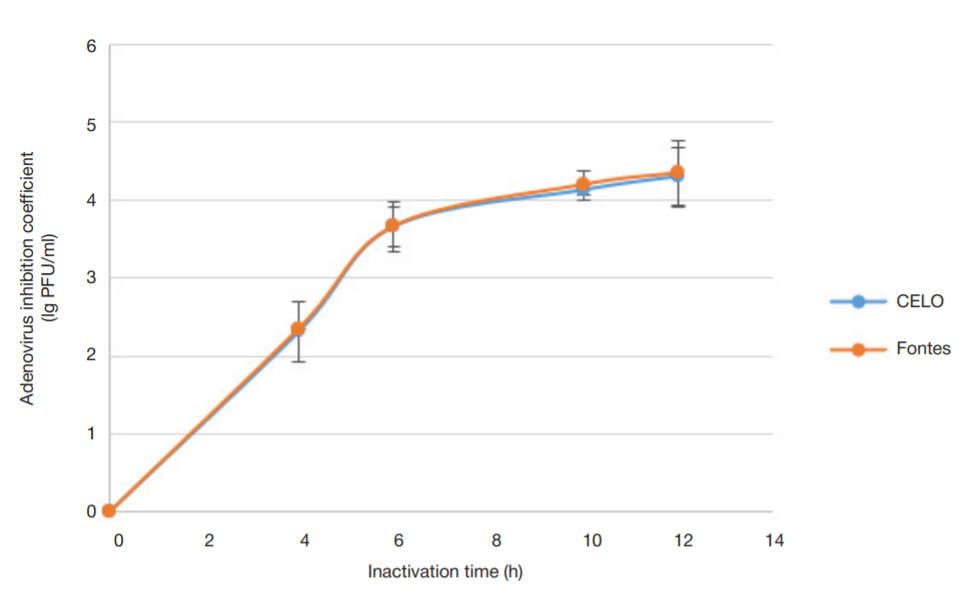
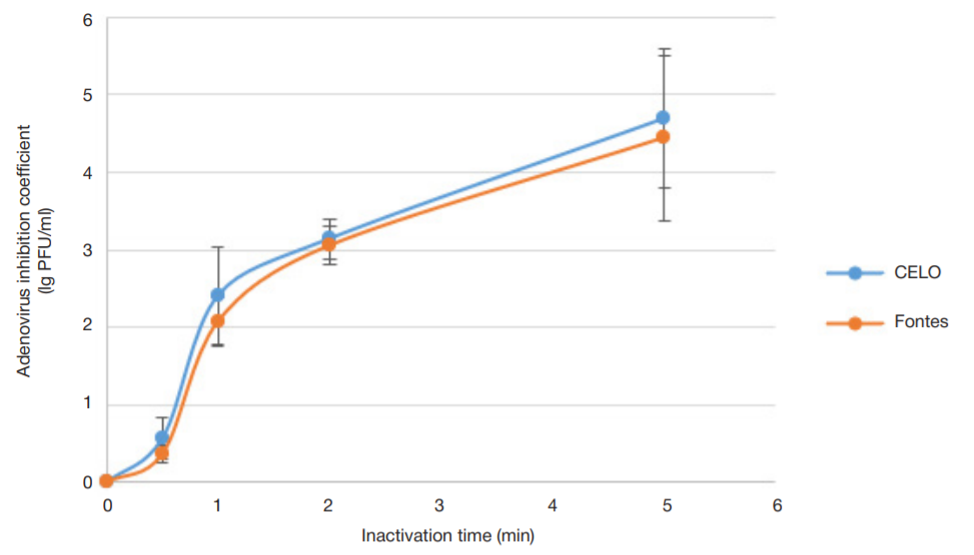
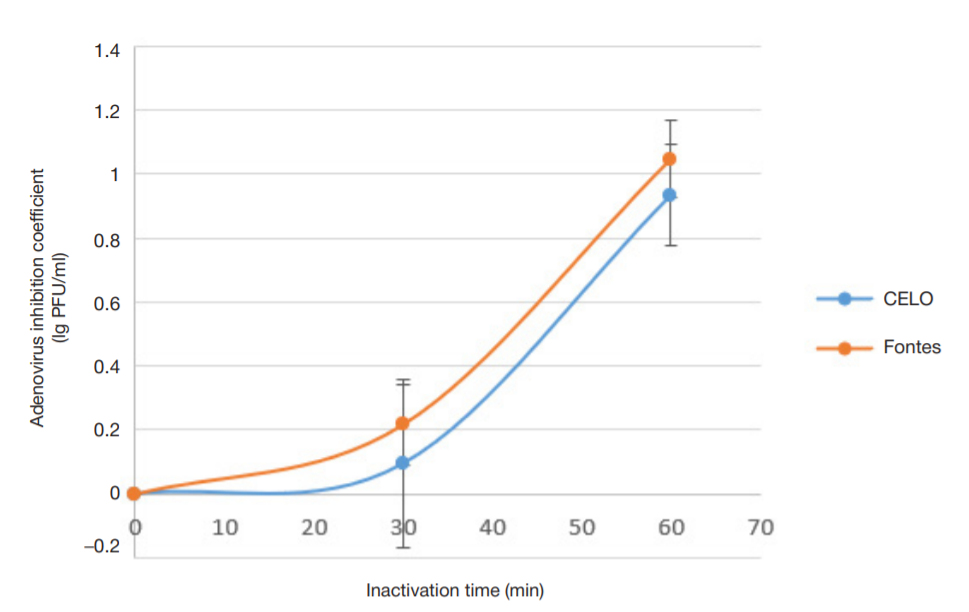
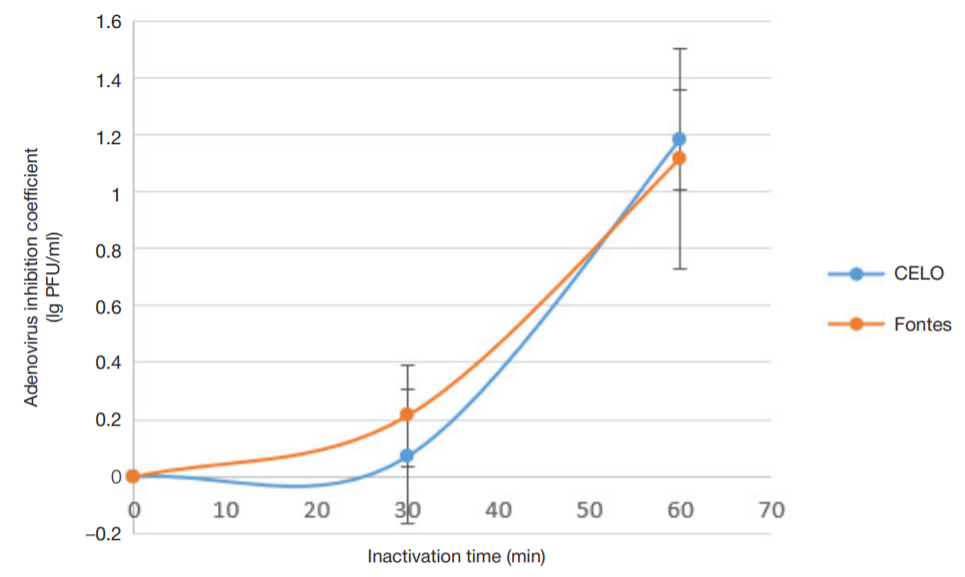
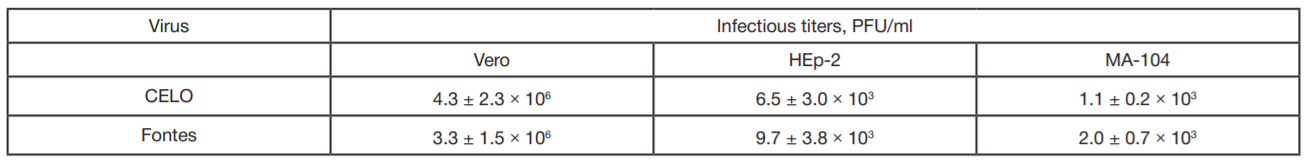
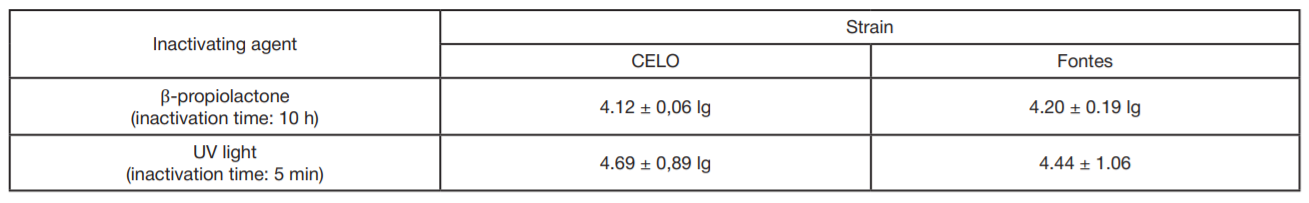
Evaluation of avian adenovirus inactivation methods used in the production of influenza vaccines
1 Saint Petersburg Research Institute of Vaccines and Serums, FMBA, Russia
2 Saint Petersburg Pasteur Research Institute of Epidemiology and Microbiology, Saint Petersburg, Russia
Correspondence should be addressed: Natalya N. Savina
Svobody, 52, Krasnoye Selo, Saint Petersburg, 198320; ur.sviinbps@anivas.n.n
Author contribution: all authors have equally contributed to the methodology of the study, analysis and interpretation of the results and manuscript preparation.
Compliance with ethical standards: the study complied with the principles of the Declaration of Helsinki (1964) and its revisions.