This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
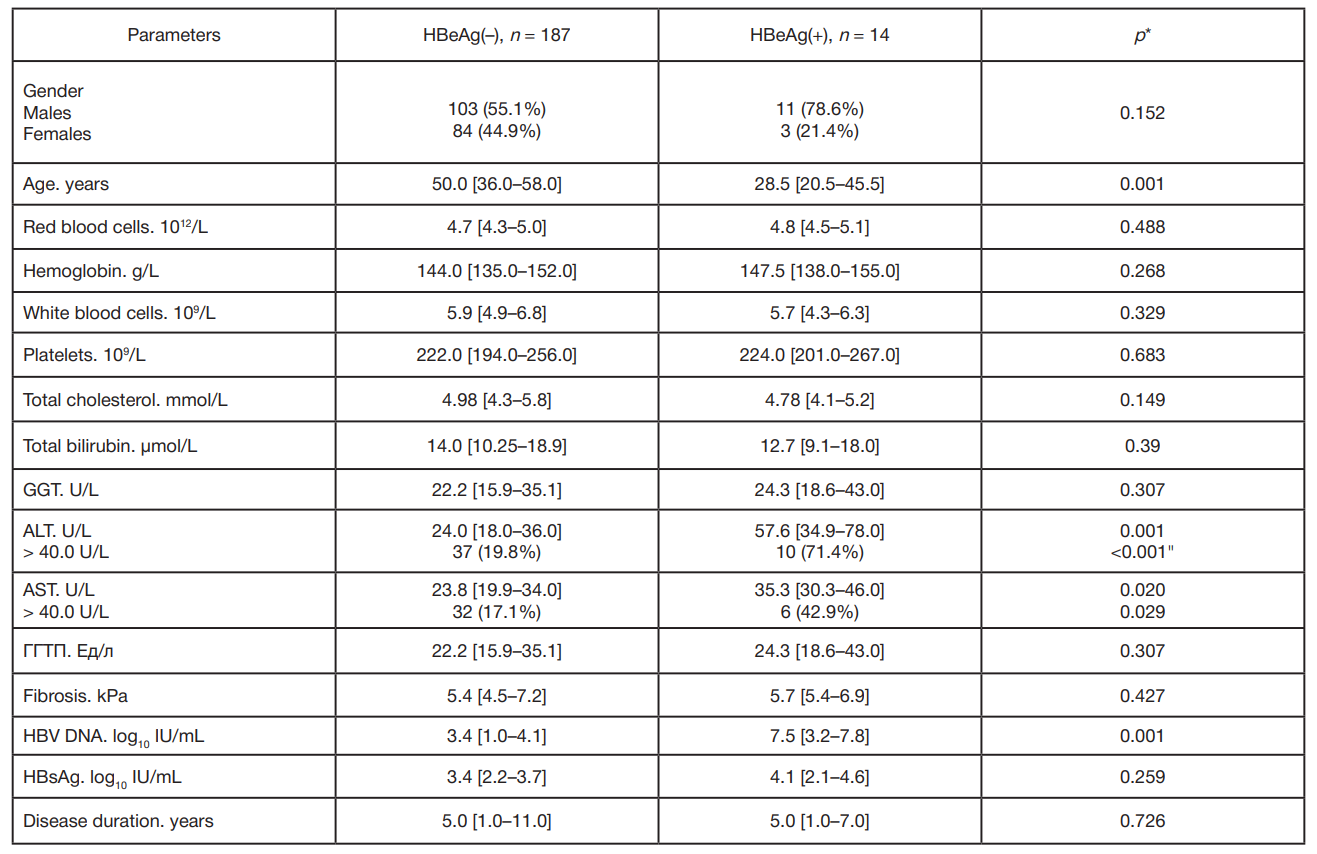
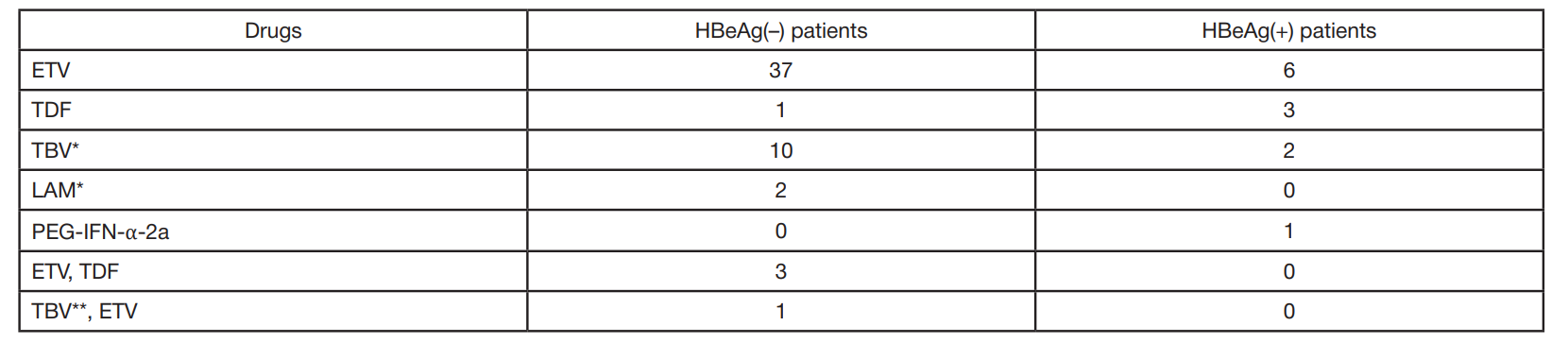
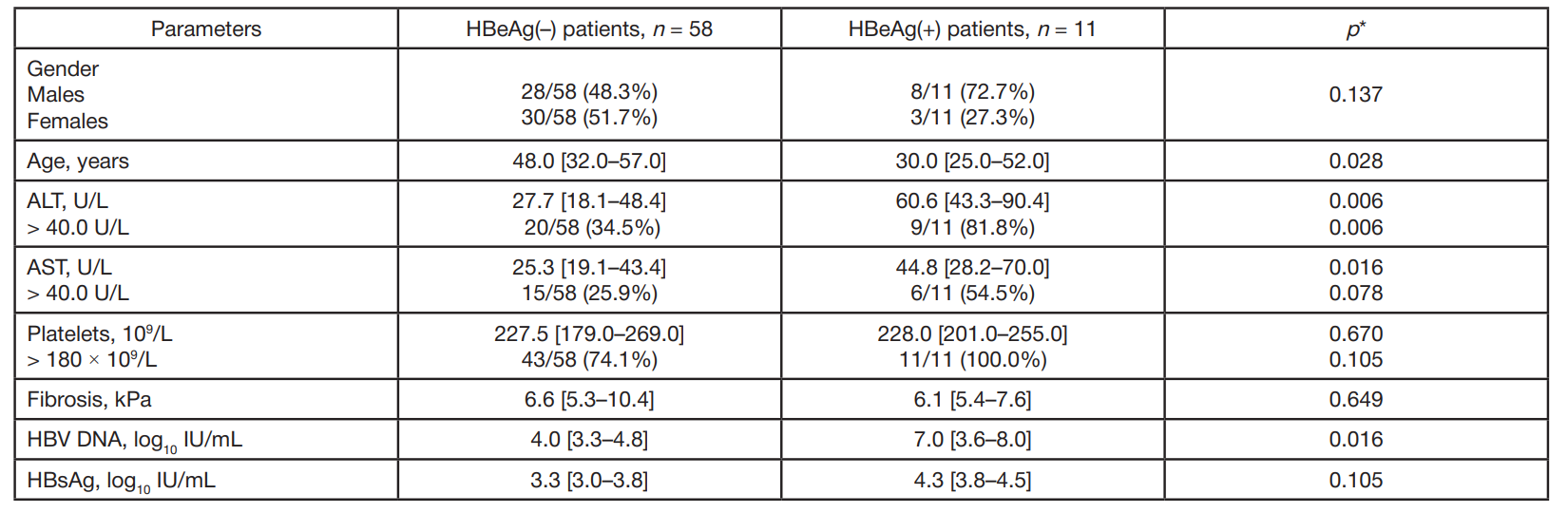
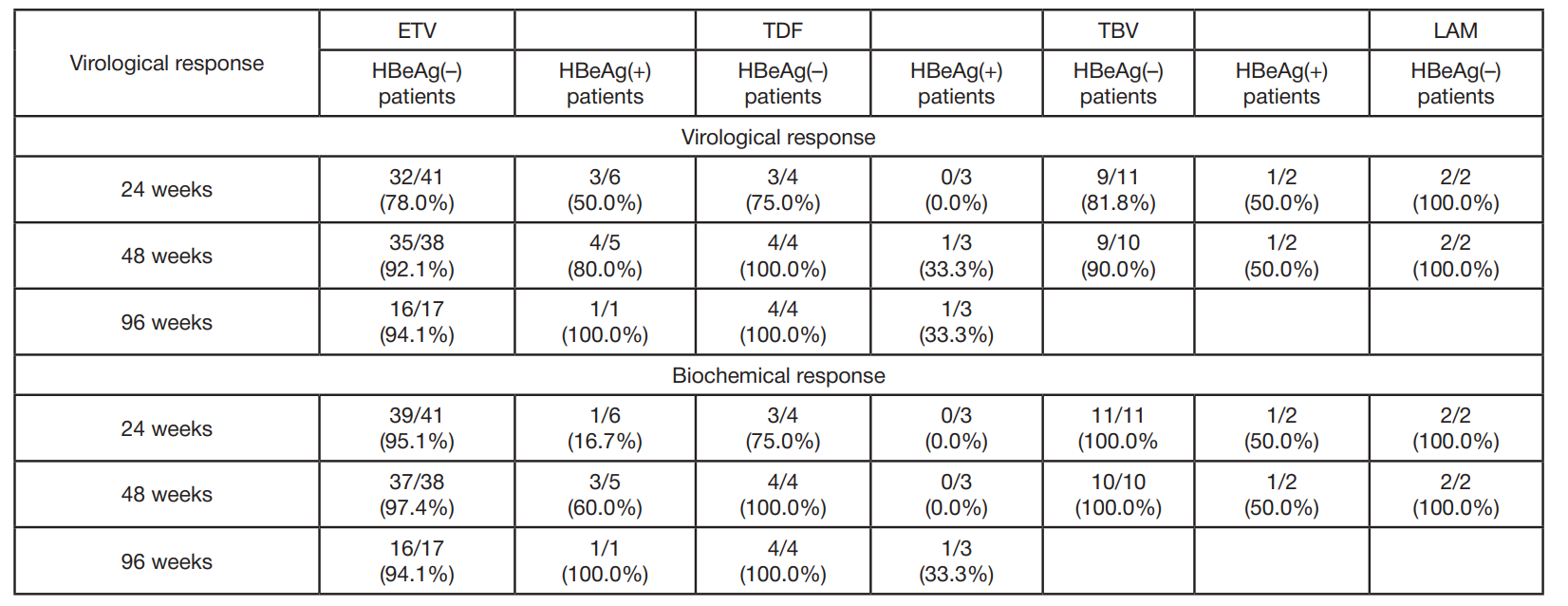
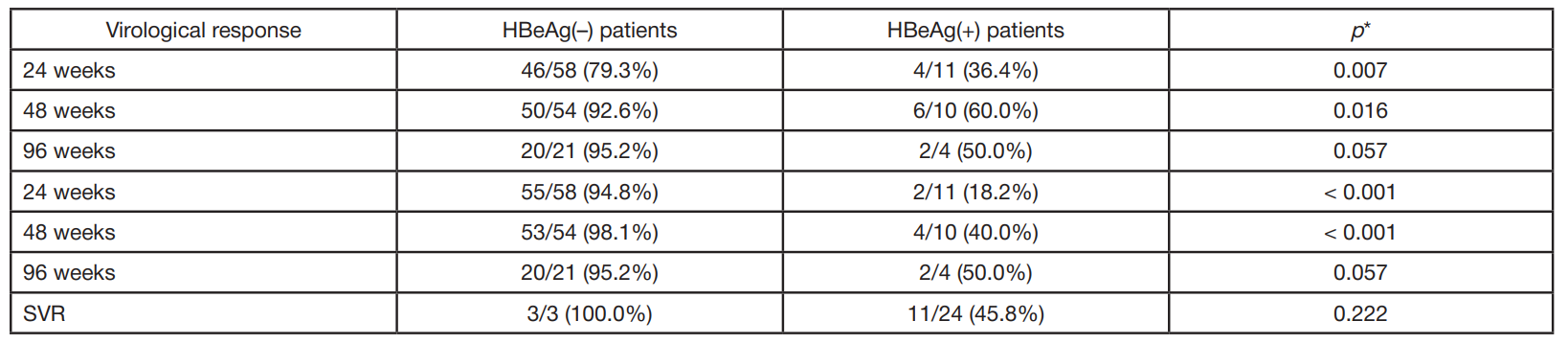
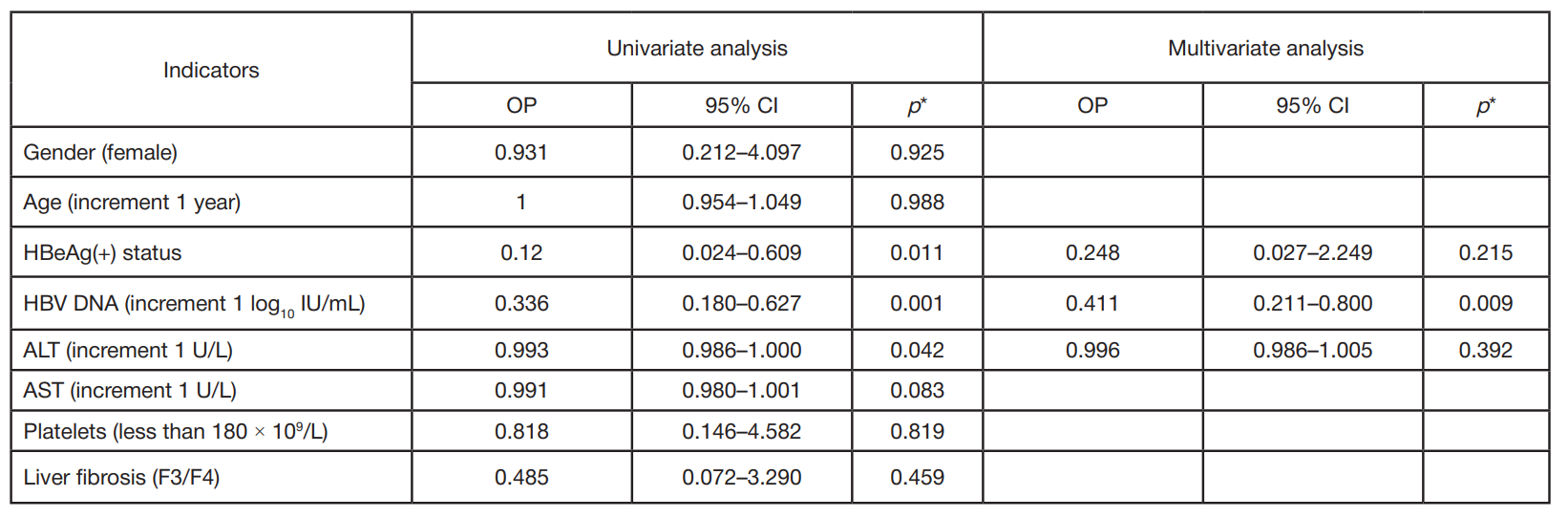
Clinical and virological characteristics of chronic hepatitis B and response to antiviral therapy
1 Pirogov Russian National Research Medical University, Moscow, Russia
2 Clinical Hospital № 85 of Federal Medical-Biological Agency, Moscow, Russia
3 Chumakov Federal Scientific Center for Research and Development of Immune-and-Biological Products, Moscow, Russia
4 Mechnikov Research Institute of Vaccines and Sera, Moscow, Russia
Correspondence should be addressed: Nguyen Thi-Hanh
Akademika Volgina, 39, Moscow, 117437, Russia; ur.liam@hnahrd
Author contribution: Nguyen Thi-Hanh — sample collection, data analysis, manuscript writing; Melnikova LI — sample collection, data analysis; Ilchenko LYu — study design, data analysis, manuscript editing; Kyuregyan KK — literature review; Gordeychuk IV — data analysis; Bondarenko NL — editing and approval of the final version of the article.
Compliance with ethical standards: the study was approved by the Ethics Committee of Pirogov Russian National Research Medical University (protocol № 213 of 13 December 2021).