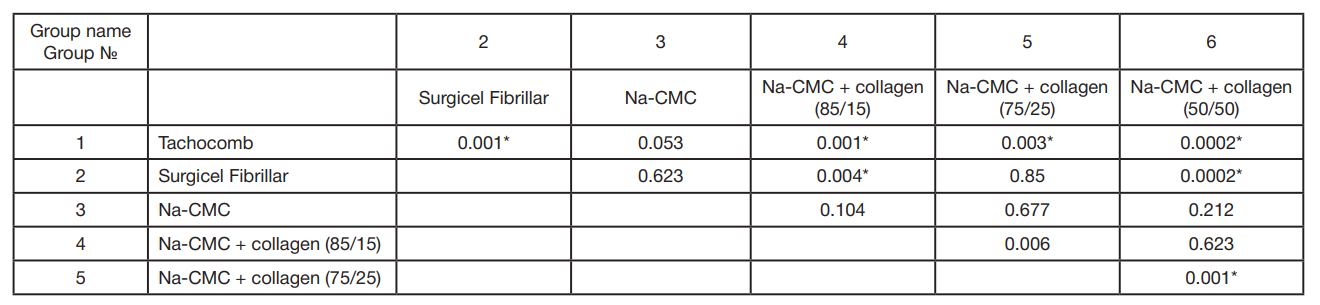
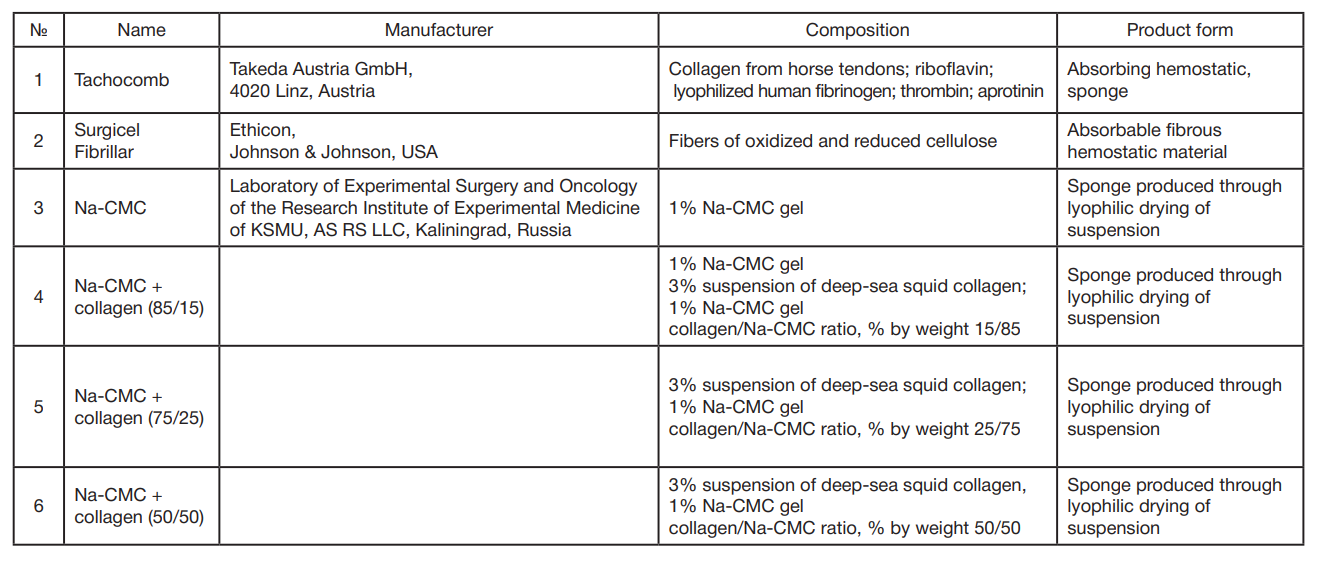
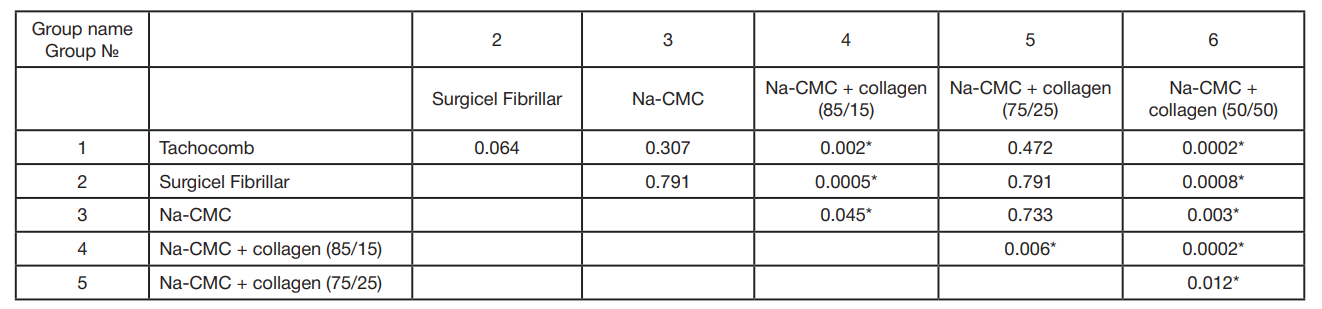
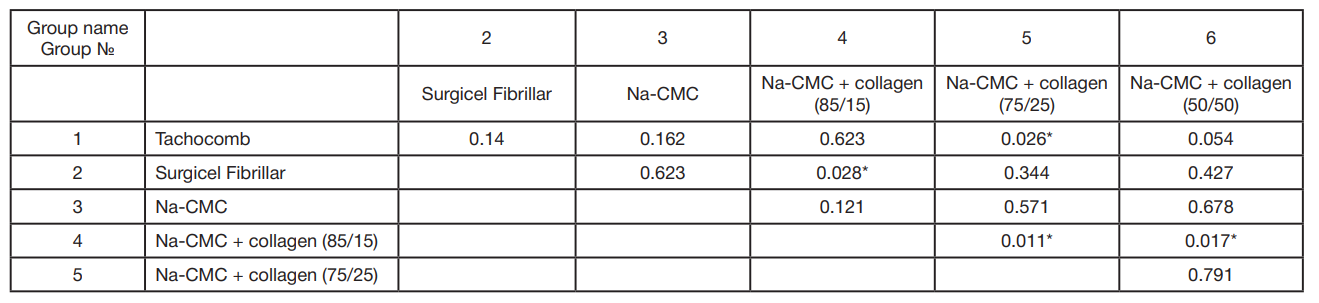
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
Comparative analysis of efficacy of the new local hemostatic agents
1 Department of operative surgery and topographic anatomy, Kursk State Medical University, Kursk, Russia
2 Immanuel Kant Baltic Federal University, Kaliningrad, Russia
3 Russian Union of Chemical Complex Enterprises and Organizations, Moscow, Russia
Correspondence should be addressed: Dmitry A. Severinov
K.Marksa, 3, Kursk, 305041, Russia; ur.liam@39.vonireves.yirtimd
Author contributions: Lipatov VA — concept and design, article authoring, editing, approval of its final version; Lazarenko SV — experimental part, statistical processing, article editing, approval of its final version; Severinov DA — experimental part, article editing, approval of its final version; Denisov AA — experimental part, article authoring, literature data analysis; Chupakhin EG, Aniskina EN — experimental part, article authoring, literature analysis.
Compliance with ethical standards: the study was approved by the Ethics Committee (Minutes #3 of November 16, 2020), conducted in compliance with international and national standards for care and use of laboratory animals.