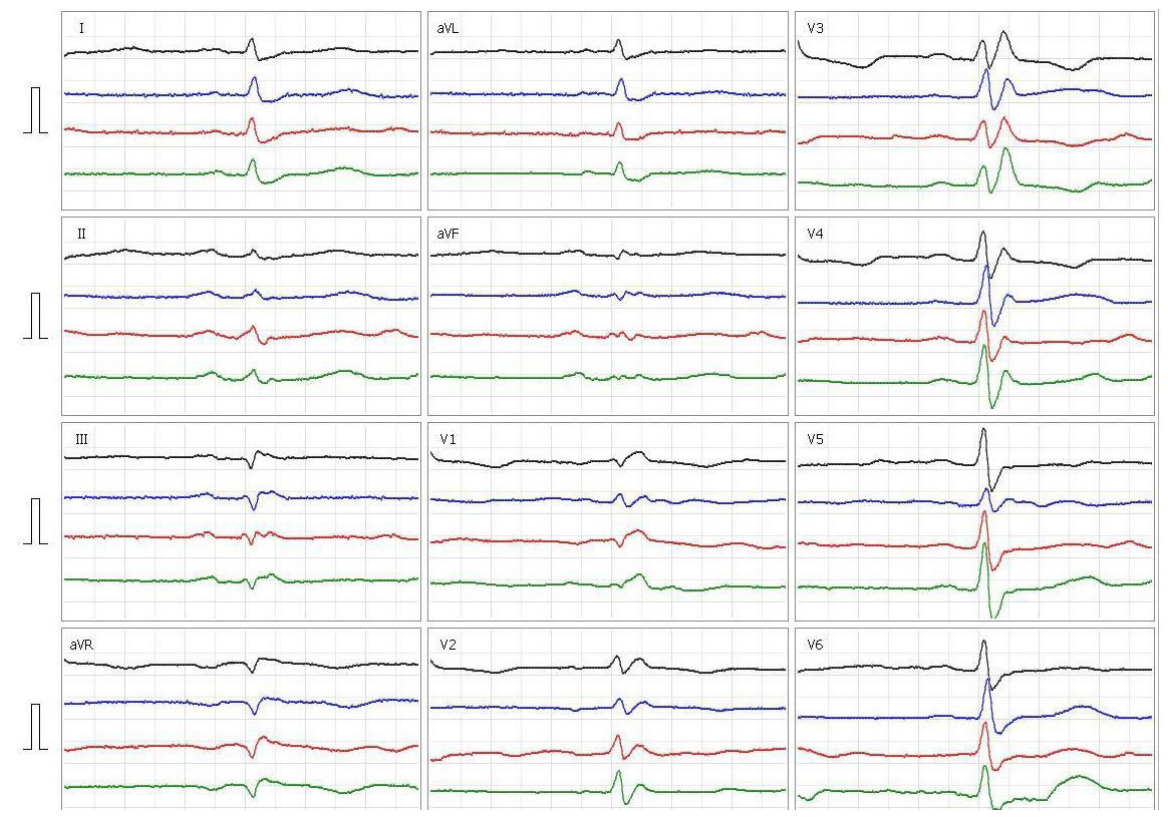
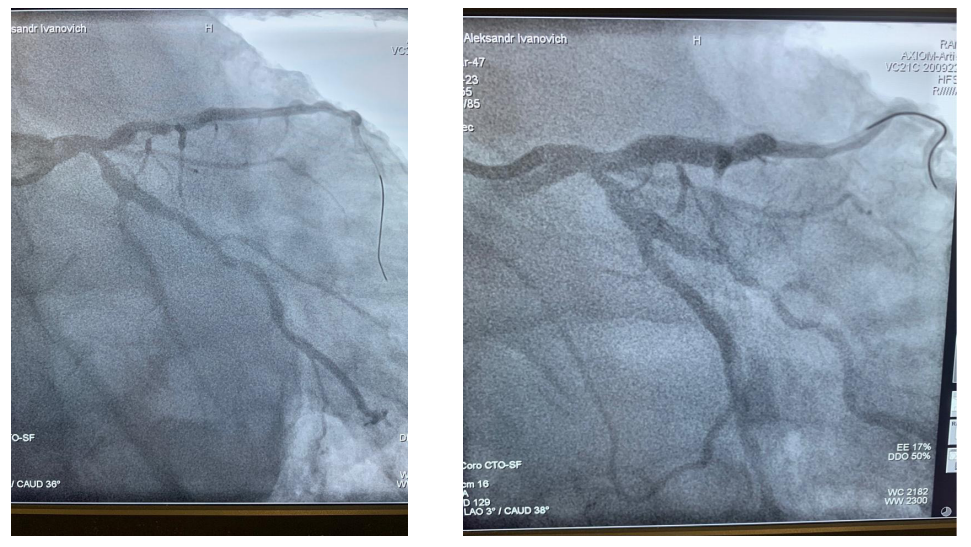
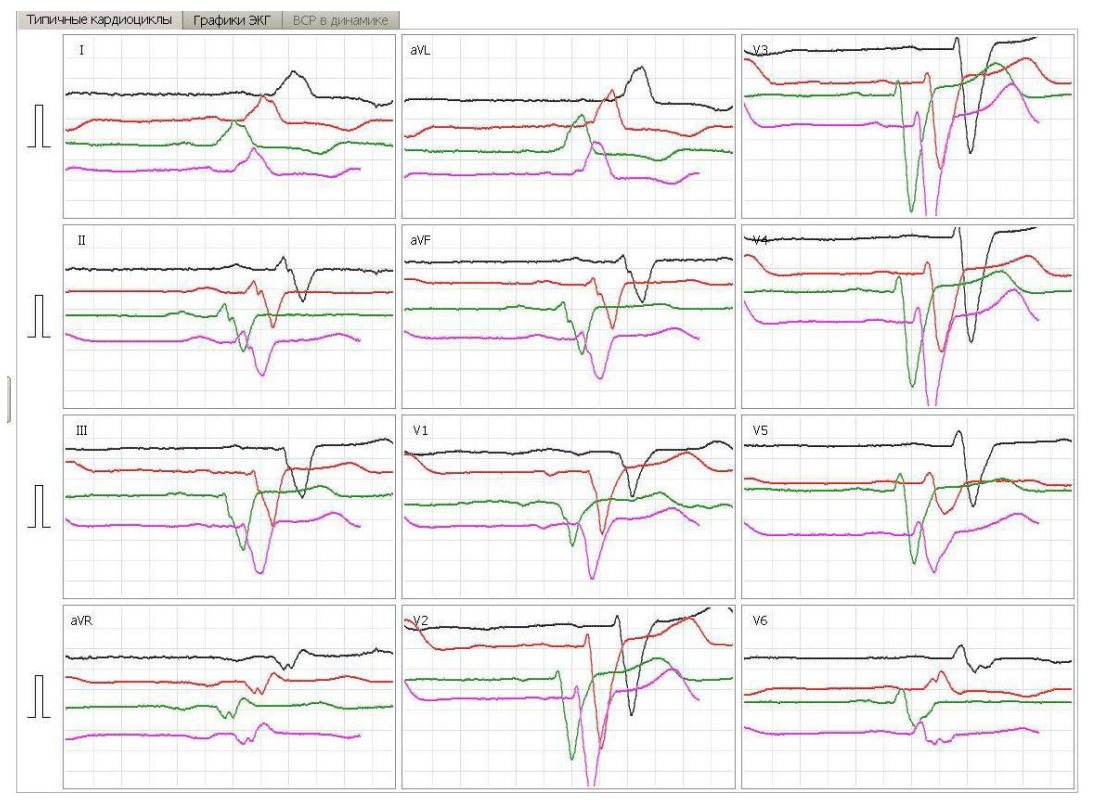
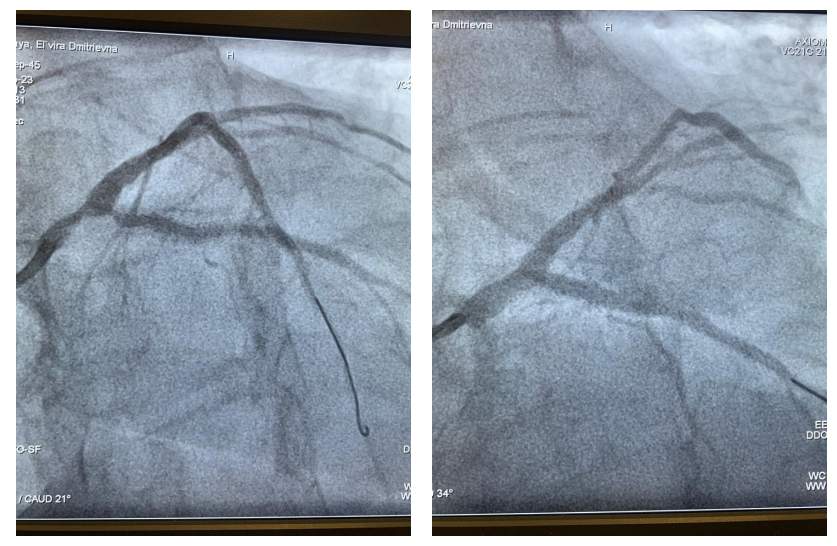
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
CLINICAL CASE
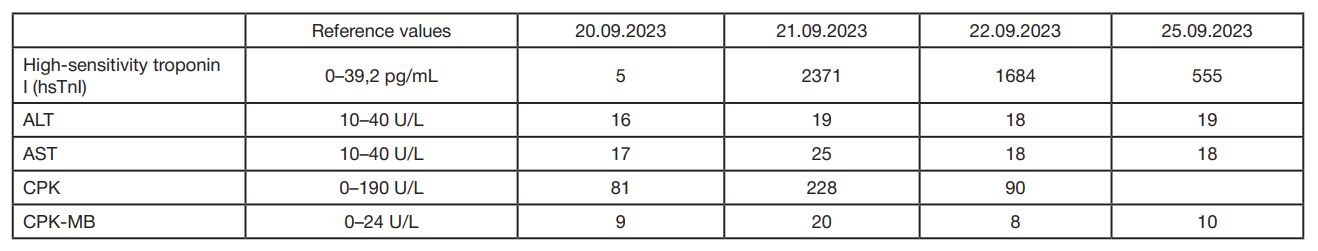
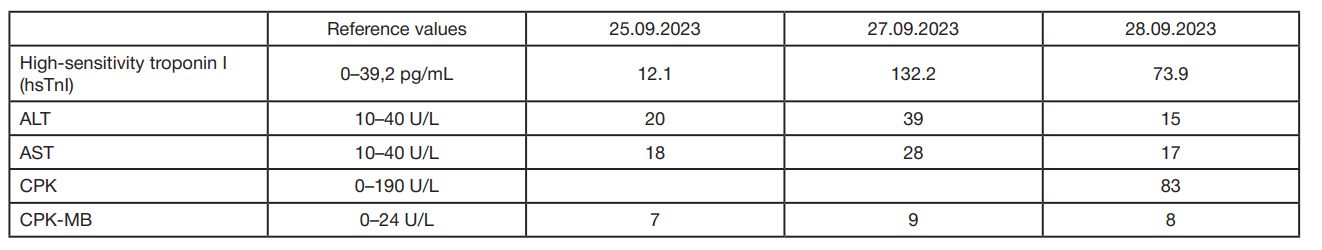
Intracoronary use of levocarnitine for coronary artery stent insertion in high-risk patients
1 Saint Petersburg State University, Saint Petersburg, Russia
2 Saint Petersburg Clinical Hospital of the Russian Academy of Sciences, Saint Petersburg, Russia
3 Clinical Hospital of the Sokolov North-Western District Scientific and Clinical Center of the Federal Medical Biological Agency, Saint Petersburg, Russia
4 Central Clinical Hospital with a Polyclinic of the Administrative Directorate of the President of the Russian Federation, Moscow, Russia
Correspondence should be addressed: Nikita Yu. Semigolovskii
pr. Kultury, 4, Saint Petersburg, 194291, Russia; ur.xednay@iksvologimes
Funding: the study was performed as part of the State Assignment No. 075-01609-23-04 “Adjuvant Cytoprotection by Intracoronary Levocarnitine Administration during Revascularization in Patients with Acute and Chronic Coronary Syndrome” (FUEM-2023-0018). Research project No. 1022040701249-2-3.2.4. Agreement No. 075-03-2023-695/1 dated 21.04.2023.
Author contribution: Semigolovskii NYu — study concept and design, text development, literature review; Kozaev AN, Nikolskaya EM, Semenova IG — сollection and processing of material; Mazurenko SO — literature review, material analysis, editing; Guslev AB, Scheglov AN — material analysis; Balluzek MF — material analysis, editing.