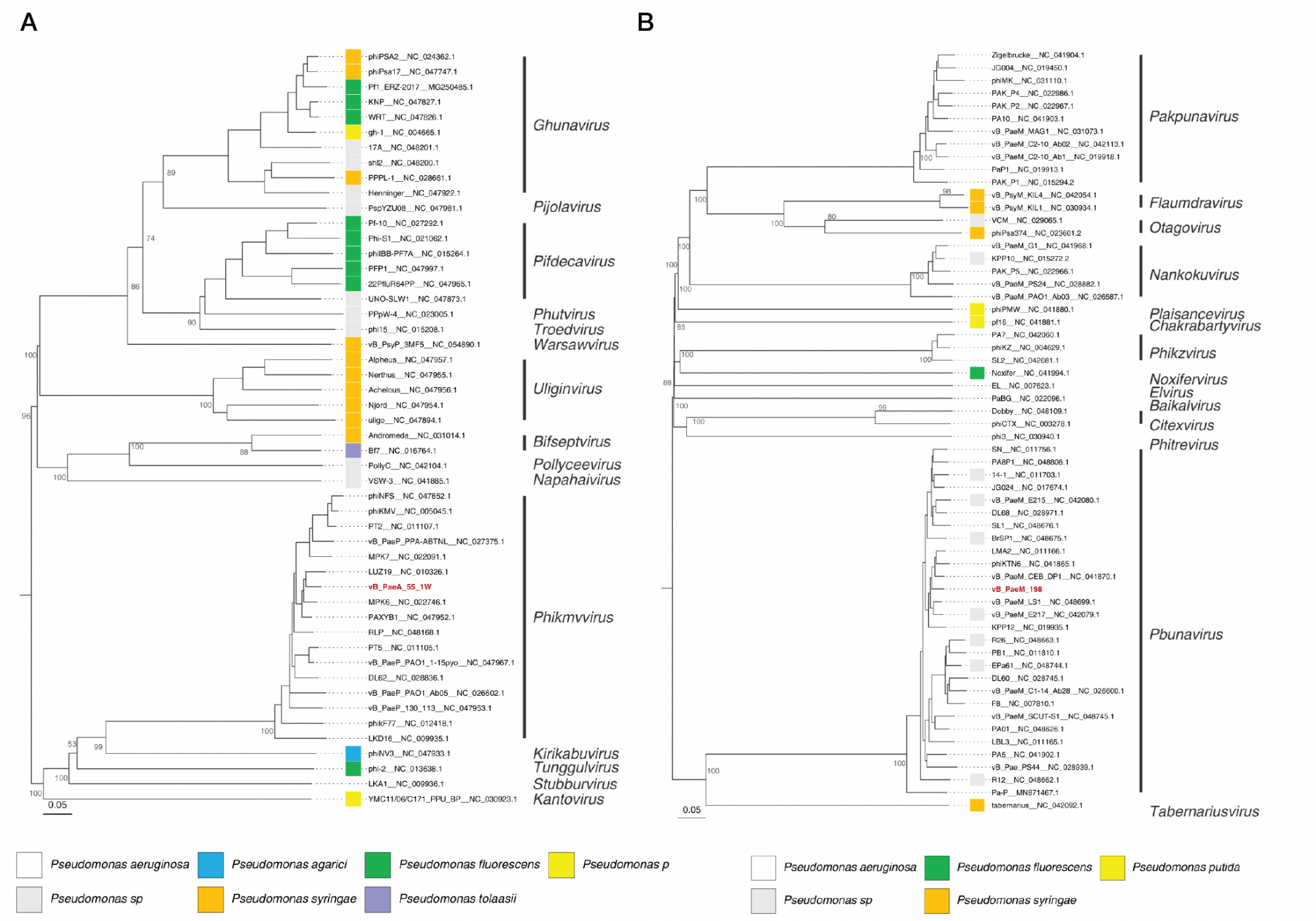
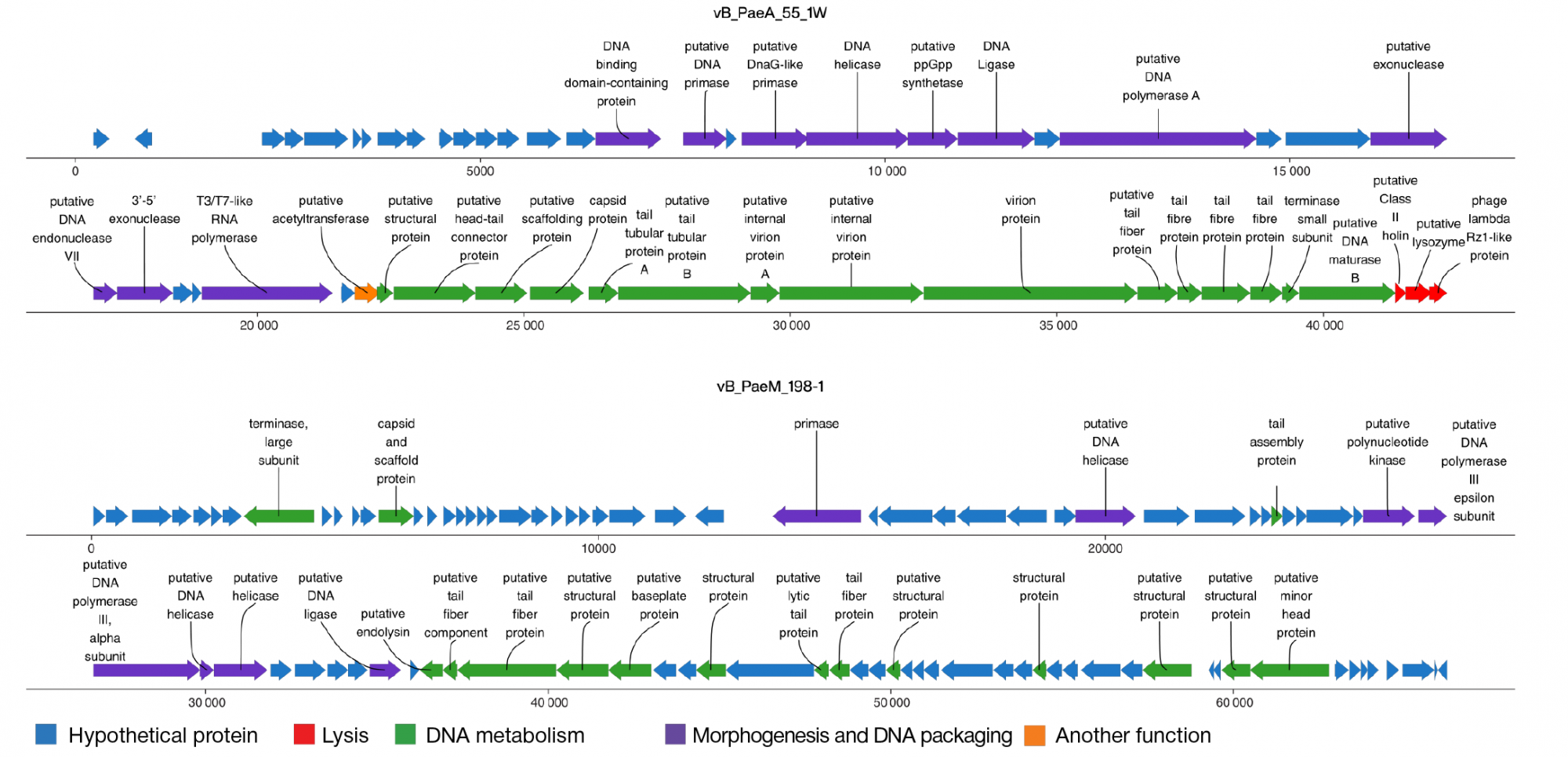
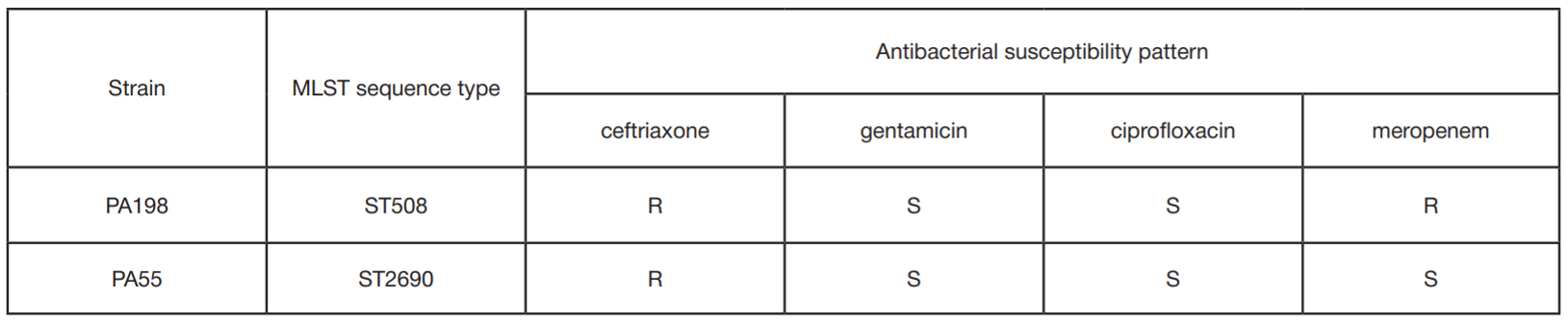
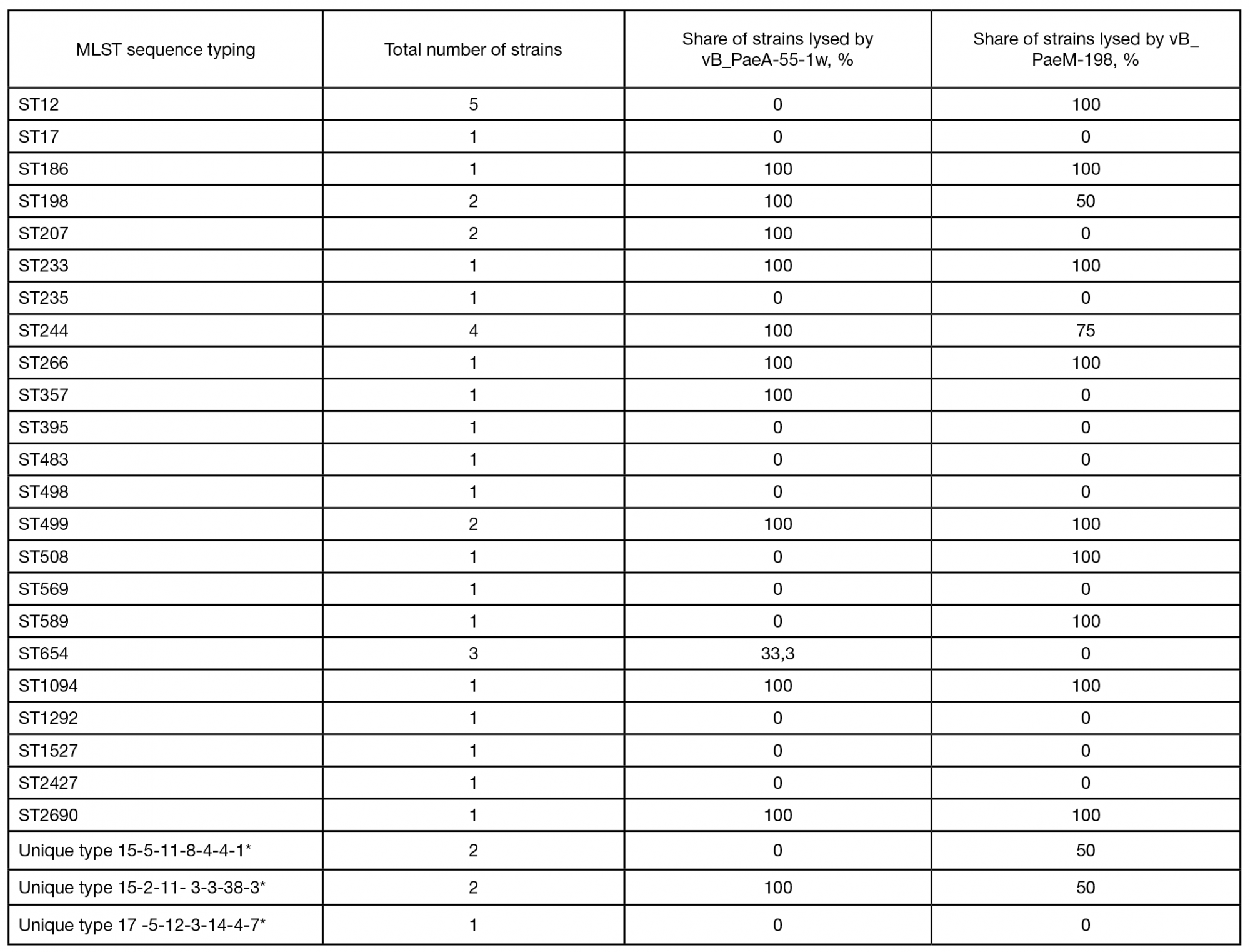
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
Isolation and characterization of Pseudomonas aeruginosa bacteriophages — potential agents for phage therapy
Federal Research and Clinical Center of Physical-Chemical Medicine of Federal Medical Biological Agency, Moscow, Russia
Correspondence should be addressed: Maria A. Kornienko
Malaya Pirogovskaya, 1a, Moscow, 119435; moc.liamg@ayiramokneinrok
Funding: The study was supported by the State Assignment on the Development of a personalized approach to the therapy of infections using virulent bacteriophages (Code: Bacteriophage) (Russia).
Acknowledgments: the authors thank the Center for Precision Genome Editing and Genetic Technologies for Biomedicine, the Federal Research and Clinical Center of Physical-Chemical Medicine of the Federal Medical Biological Agency for their help with sequencing the genomes of bacteriophages.
Author contribution: Kornienko MA — study plan, data collection and processing, article authoring; Kuptsov NS — data collection and processing, article authoring; Danilov DI, Gorodnichev RB, Malakhova MV, Veselovsky VA — data collection; Bespiatykh DA — data processing, Shitikov EA — research plan, data processing, article authoring; Ilina EN — research plan, article authoring.
Compliance with ethical standards: the experiment was carried out in compliance with the Sanitary and Epidemiological Rules SP 1.3.2322-08 "Safe work with microorganisms of III–IV pathogenicity (hazardousness) groups and pathogens of parasitic diseases"; Sanitary and Epidemiological Rules SP 1.3.2518-09 "Amendments and additions #1 to the sanitary and epidemiological rules" Safe work with microorganisms of III–IV pathogenicity (hazardousness) groups and pathogens of parasitic diseases"; Sanitary and Epidemiological Rules SanPiN 2.1.7.2790-10 "Sanitary and epidemiological requirements for medical waste management"; Federal Clinical Recommendations "Rational use of bacteriophages in medical and anti-epidemic practice."