This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
METHOD
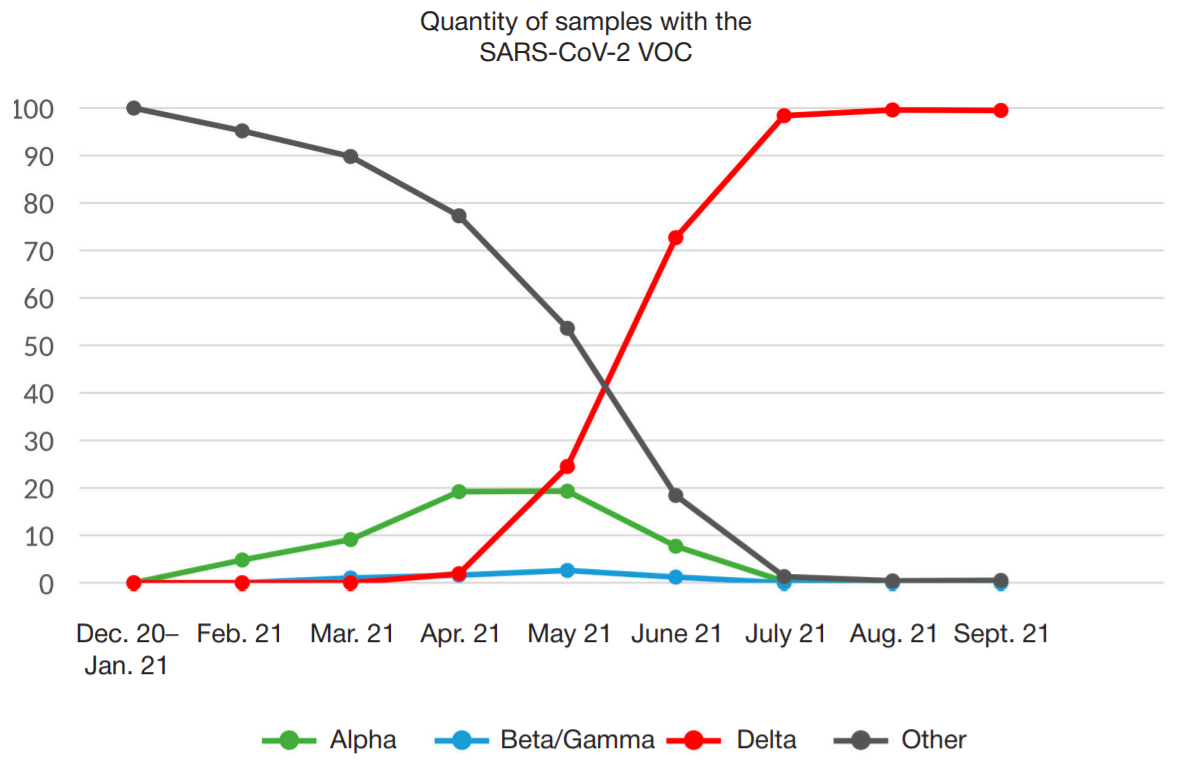
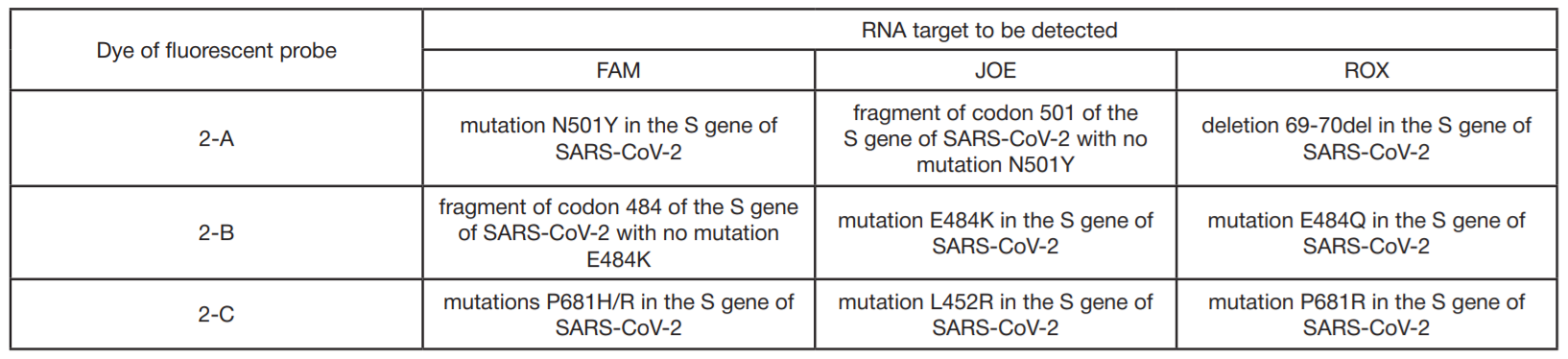
Development of PCR test for detection of the SARS-CoV-2 genetic variants alpha, beta, gamma, delta
Centre for Strategic Planning and Management of Biomedical Health Risks of Federal Medical Biological Agency, Moscow, Russia
Correspondence should be addressed: Anna K. Shuryaeva
Pogodinskaya, 10, str. 1, 119121, Moscow; ur.zmpsc@aveayruhsa
Author contribution: Shipulin GA, Savochkina YuA, Davydova EE, Yudin SM — planning the experiment; Shipulin GA, Savochkina YuA, Yudin SM — literature analysis; Shuryaeva AK, Shivlyagina EE, Nosova AO, Luparev AR, Malova TV — experimental procedure, data interpretation; Savochkina YuA, Shivlyagina EE, Nosova AO — reagent kit development; Luparev AR — statistical analysis; Shipulin GA, Shuryaeva AK — manuscript writing and editing; Davydova EE, Yudin SM — manuscript editing.
Compliance with ethical standards: the study was performed in accordance with the requirements of the Declaration of Helsinki and GOST R ISO 14155-2014.