This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
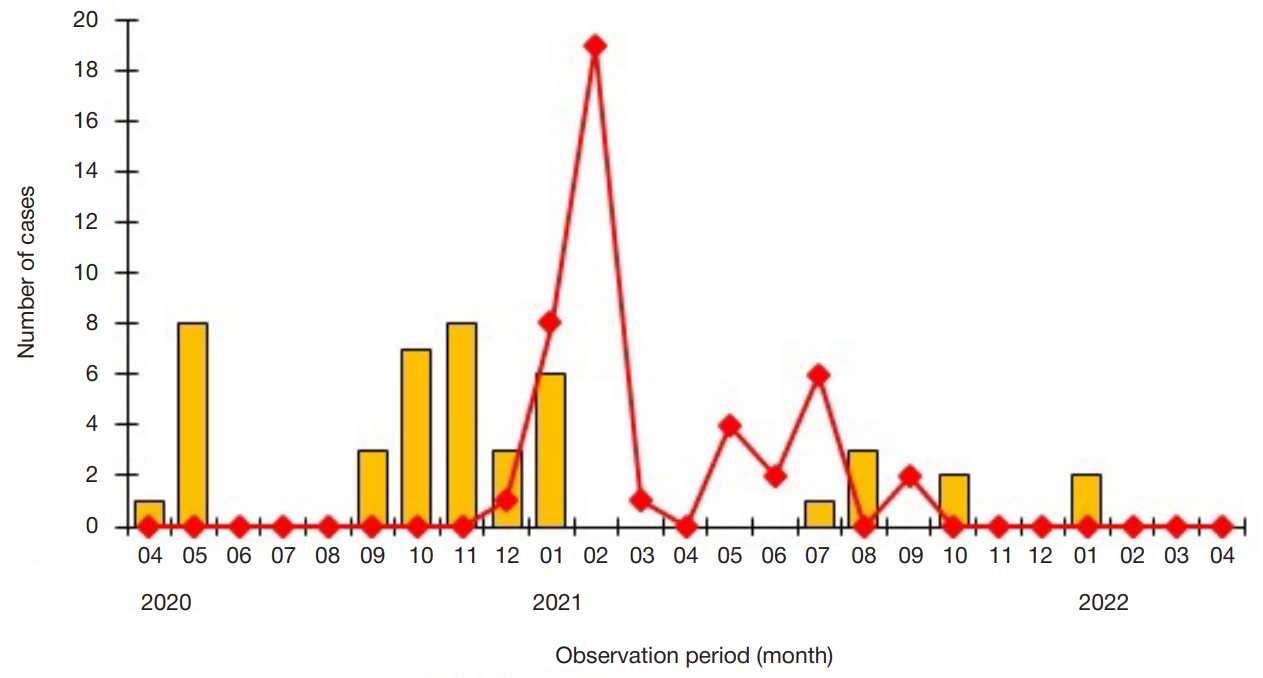
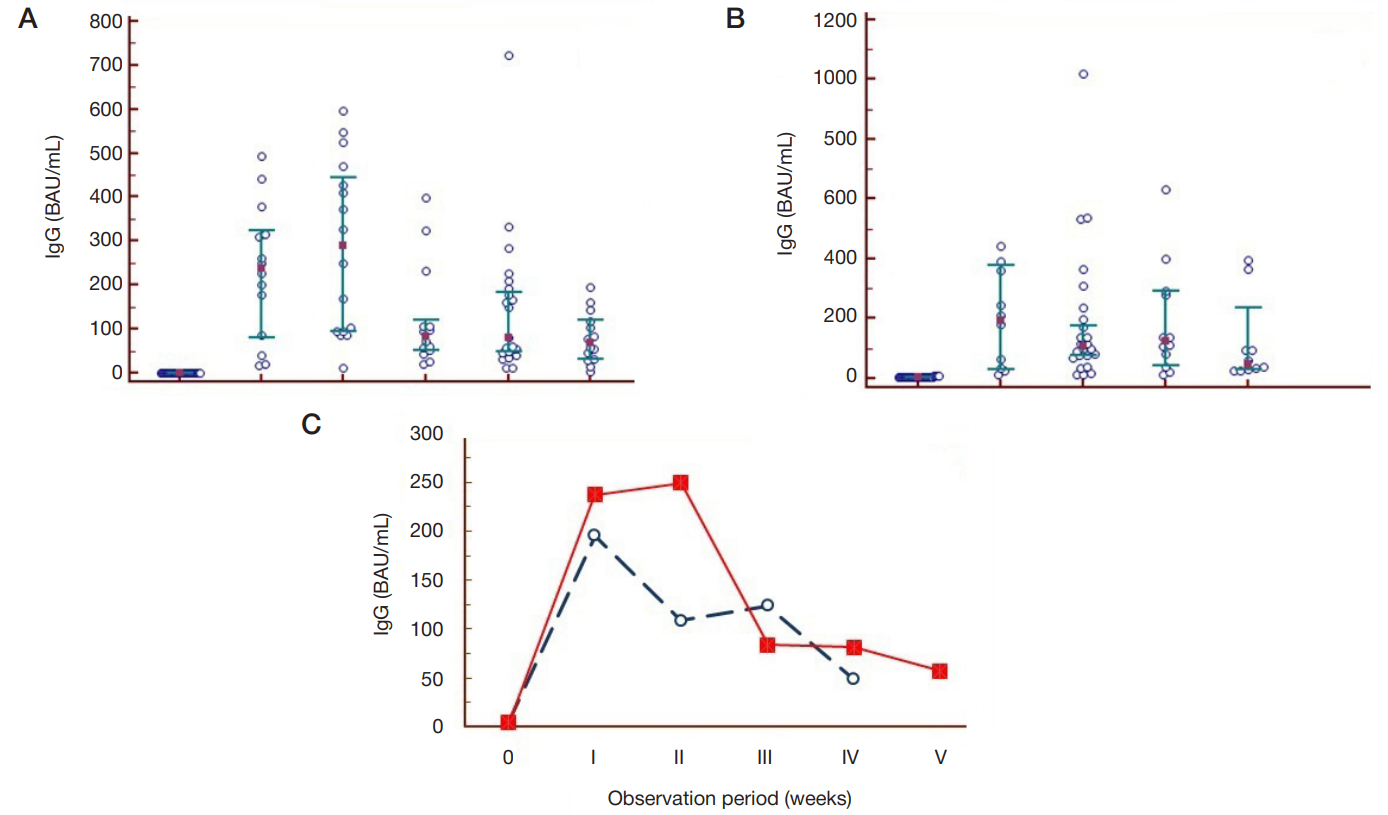
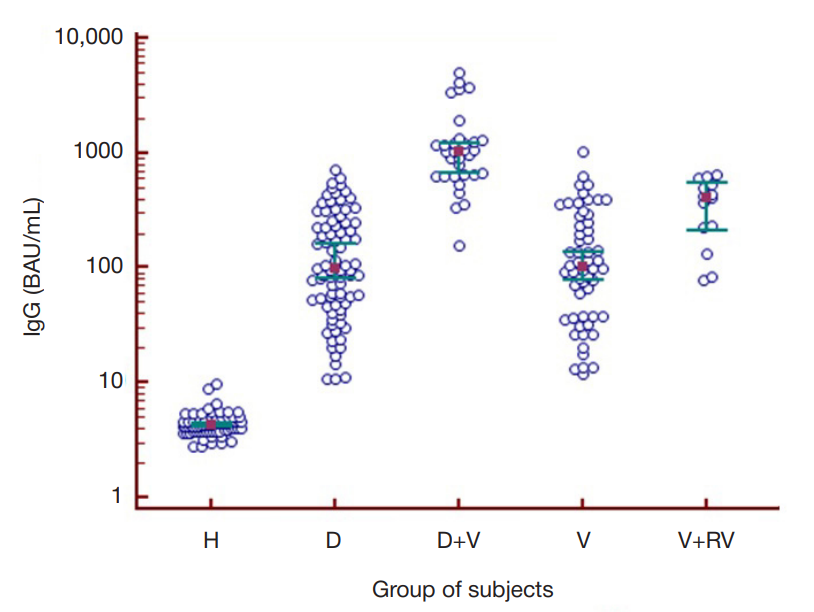
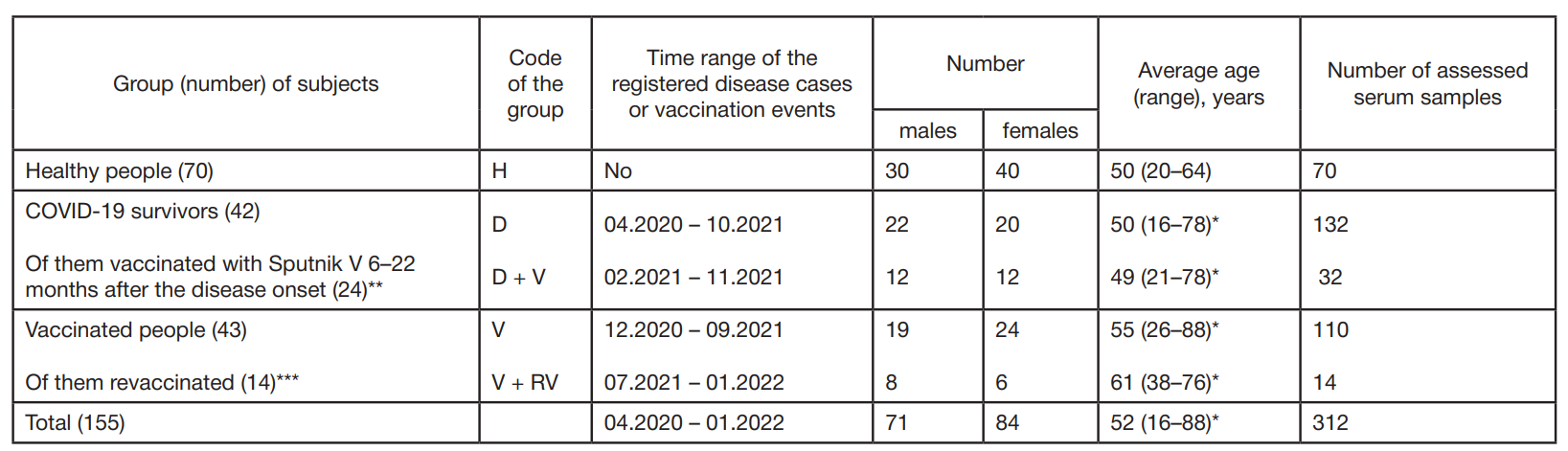
Dynamics of humoral immunity to SARS-CoV-2 in the professionally homogeneous group of people over a two-year period of COVID-19 outbreak
State Research Institute of Biological Instrumentation of the Federal Medical Biological Agency, Moscow, Russia
Correspondence should be addressed: Vera G. Pomelova
Volokolamskoe shosse, 75, corp. 1, Moscow, 125424, Russia; ur.neercsonummi@avolemop.v
Acknowledgements: we would like to thank staff members of the State Research Institute of Biological Instrumentation: Kanaeva TA for carrying out immunochipbased analysis, Balaban AS for antigen printing with nanoplotter and immunochip preparation for analysis, Stadnik OB for management of the serum samples acquisition and testing using commercially available immunoassay kit.
Author contribution: Pomelova VG — concept, experimental design, manuscript writing; Bychenkova TA — management of immunochip-based immunochemistry studies, data processing; Bekman NI — statistical analysis, preparation of illustrations; Osin NS — providing technical assistance, data analysis and discussion, manuscript editing; Ishkov YuN, Styazhkin KK — data analysis and discussion, manuscript editing.
Compliance with ethical standards: the study was approved by the Ethics Committee of the State Research Institute of Biological Instrumentation (protocol № 4 dated June 09, 2021). The informed consent was submitted by all subjects.