This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
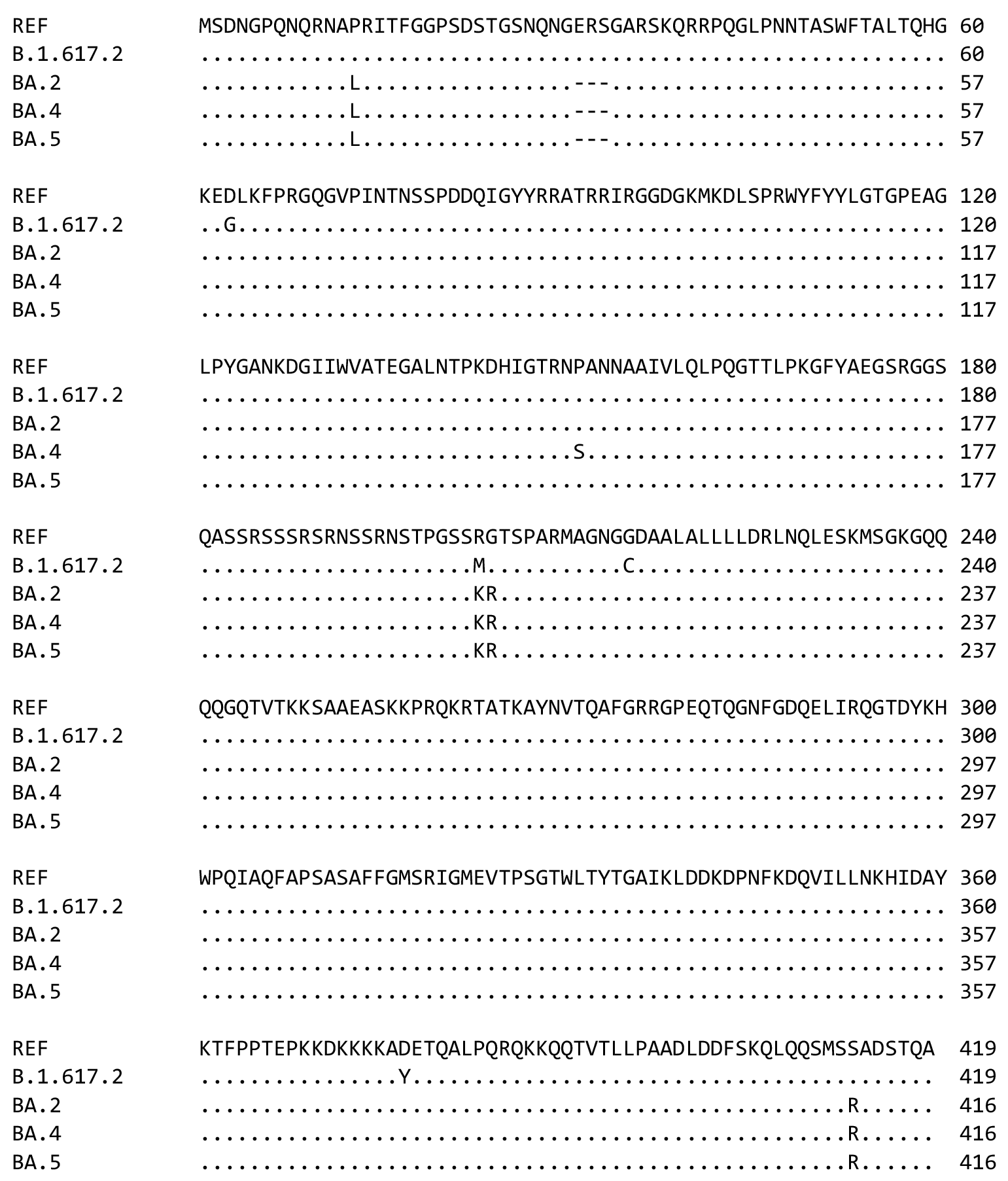
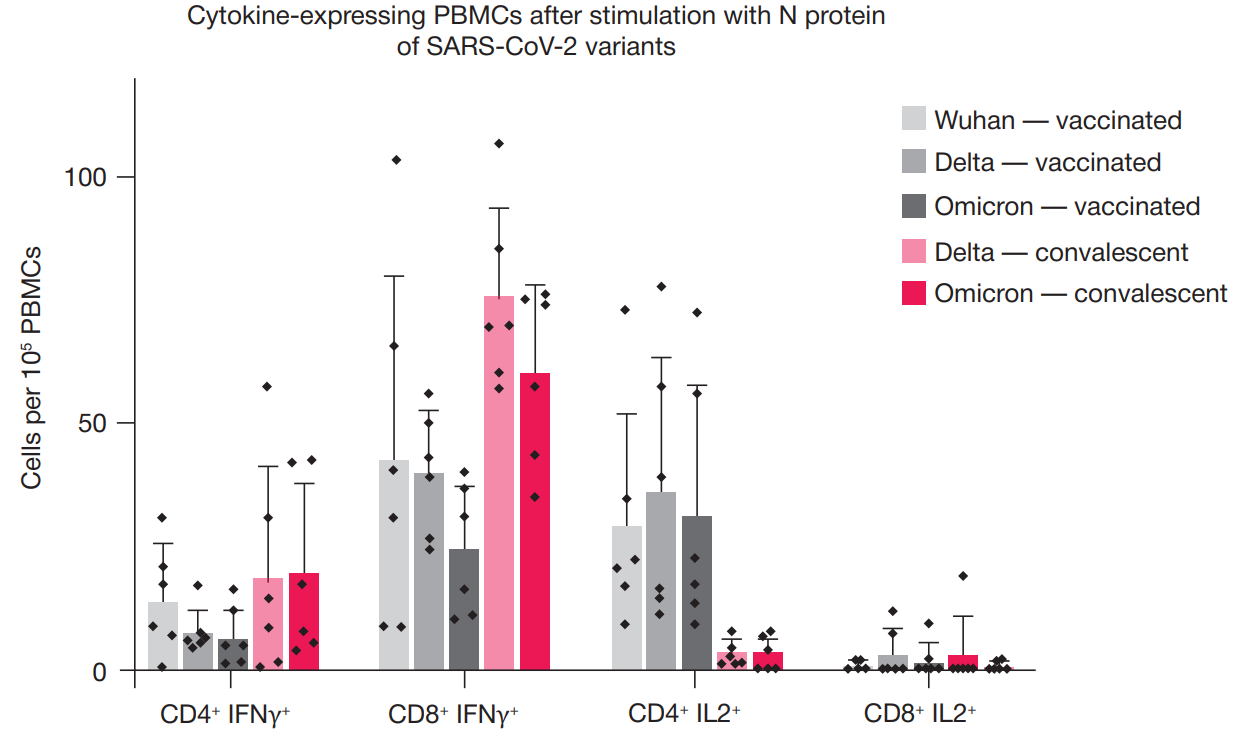
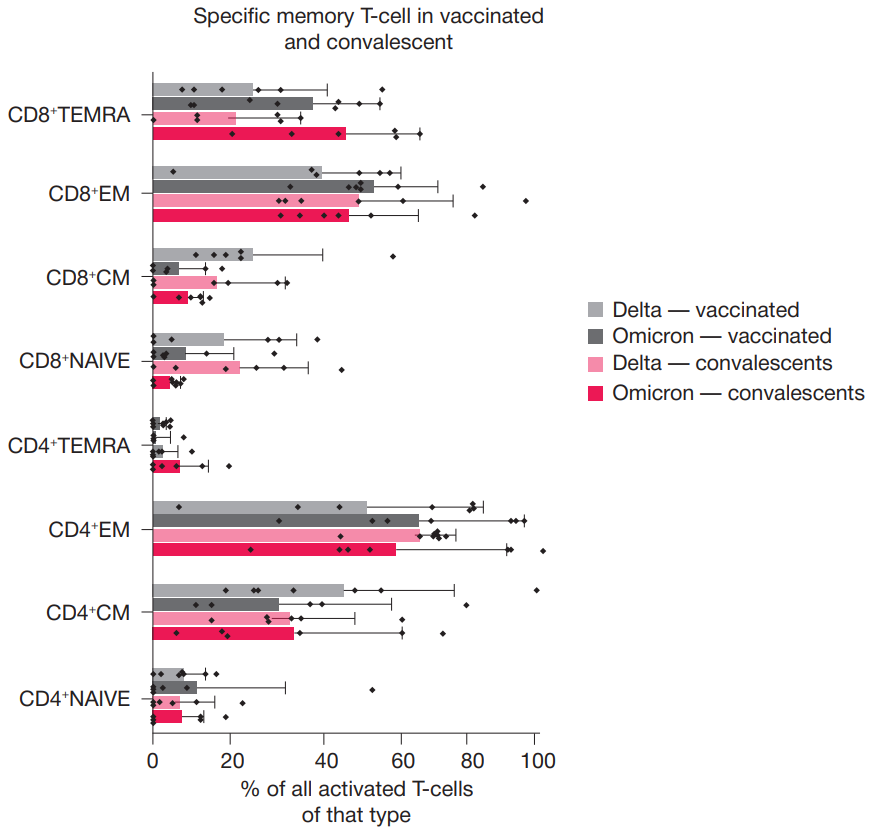
N protein based vaccine against SARS-CoV-2 produces a strong T cell immune response to N Protein of novel strains
1 Saint Petersburg Scientific Research Institute of Vaccines and Serums and Enterprise for the Production of Bacterial Preparations of the Federal Medical Biological Agency, Saint Petersburg, Russia
2 Centre for Strategic Planning and Management of Biomedical Health Risks of the Federal Medical Biological Agency, Moscow, Russia
3 Institute of Immunology of the Federal Medical Biological Agency, Moscow, Russia
4 Pirogov Russian National Research Medical University, Moscow, Russia
5 Federal Medical Biological Agency, Moscow, Russia
Correspondence should be addressed: Sevastyan O. Rabdano
Svobody, 52, 198320, Krasnoye Selo, Saint Petersburg; ur.onadbar@naytsaves
Author contribution: Rabdano SO — study design, data analysis, data interpretation, manuscript writing; Mukhin VE — bioinformatic analysis, experimental procedure, data acquisition, statistical analysis, manuscript writing; Makarov VV — study design, data interpretation, manuscript writing; Rudakov GO — data analysis, statistical analysis, data interpretation, graphics preparation, manuscript writing; Ruzanova EA, Arakelov SA, Khaitov MR, Yudin SM, Kryuchko DS, Berzin IA, Evtushenko AE — study design, manuscript editing; Truhin VP, Skvortsova VI — research idea, study concept, manuscript editing.
Compliance with ethical standards: the study was approved by the Ministry of Health of the Russian Federation (clinical trial approval № 388 of 19 July 2021), Ethics Committee of MH RF (protocol № 282 of 19 July 2021) and Independent Ethics Committee (IEC) of the research center (protocols № 163 of 15 July 2021 and № 164 of 20 July 2021); the study was conducted in accordance with the principles of the World Medical Association (WMA) Declaration of Helsinki (1964) and its latest update (2013), tripartite guideline for Good Clinical Practice approved by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) (E6 (R2) of 09 November 2016) and current legislation of the EEU and RF. Two copies of the informed consent form (volunteer information sheet) were to be signed and dated by the subjects and the researcher by their own handwriting.