This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
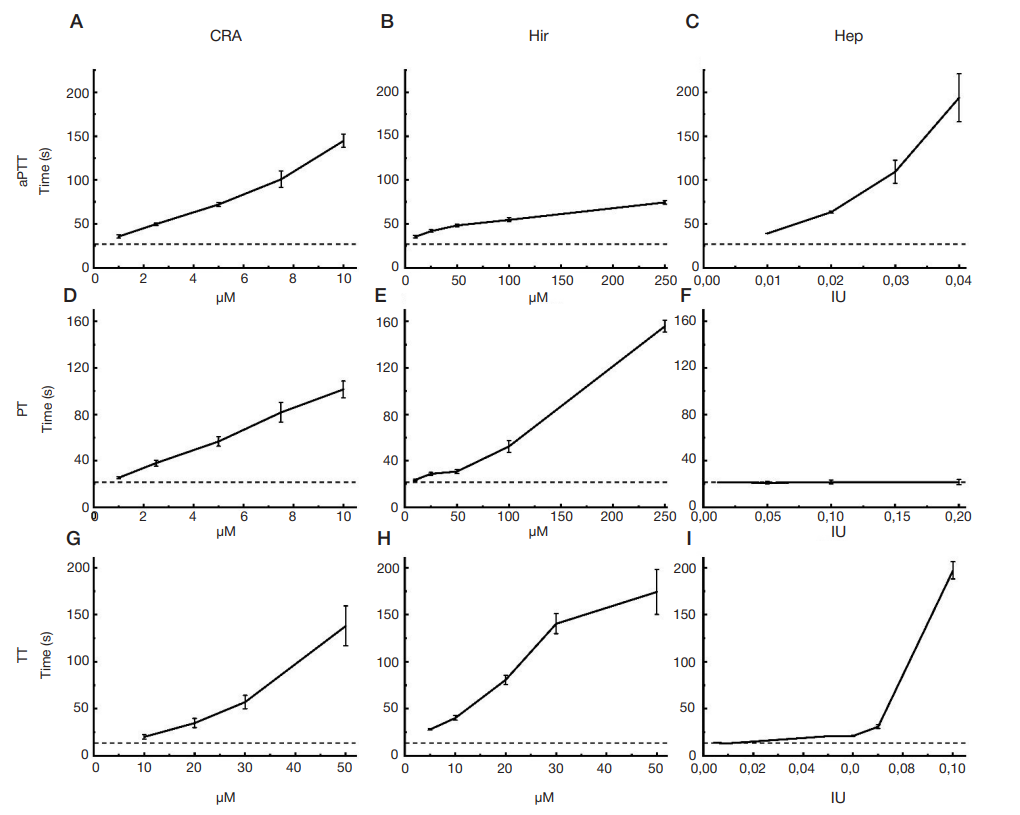
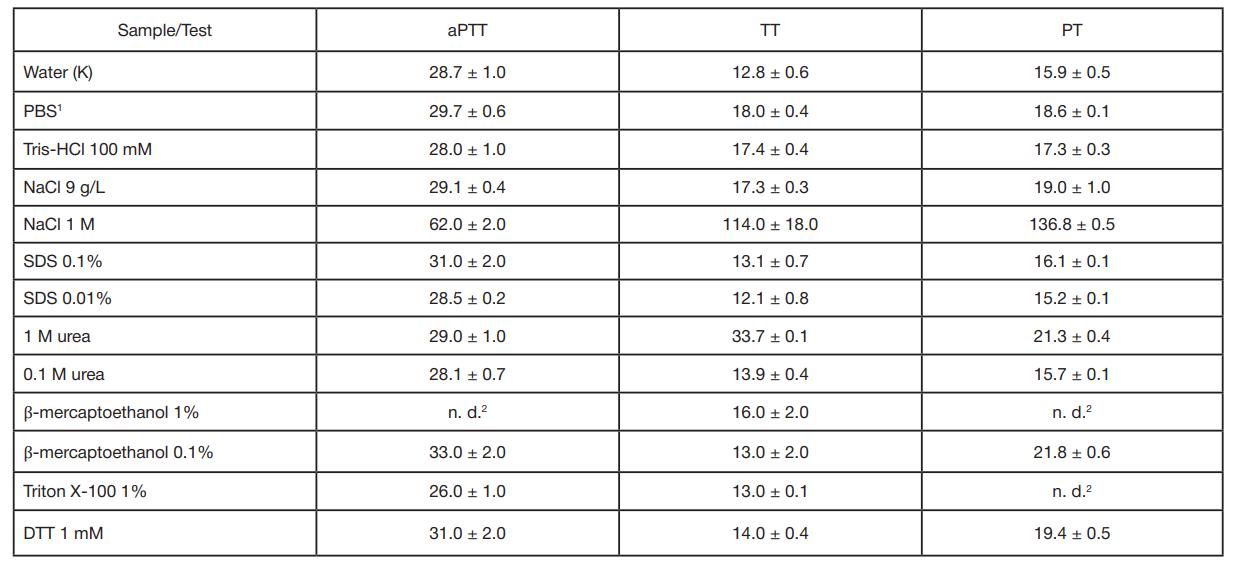
Method to assess the effects of bioactive compounds solutions on blood clotting
1 Lopukhin Federal Research and Clinical Center of Physical-Chemical Medicine of the Federal Medical Biological Agency, Moscow, Russia
2 Moscow Institute of Physics and Technology (National Research University), Dolgoprudny, Moscow Region, Russia
Correspondence should be addressed: Valentin A. Manuvera
Malaya Pirogovskaya, 1A, Moscow, 119435, Russia; ur.xednay@arevunamv
Funding: the study was supported by the Russian Science Foundation grant No. 23-25-00006, https://rscf.ru/project/23-25-00006/.
Author contribution: Manuvera VA — concept, experiments, manuscript writing; Brovina KA — experiments, manuscript editing; Bobrovsky PA — data analysis, visualization, manuscript editing; Grafskaia EN — experiments, manuscript editing; Kharlampieva DD — experiments, manuscript editing; Lazarev VN — research team management, manuscript editing.