This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
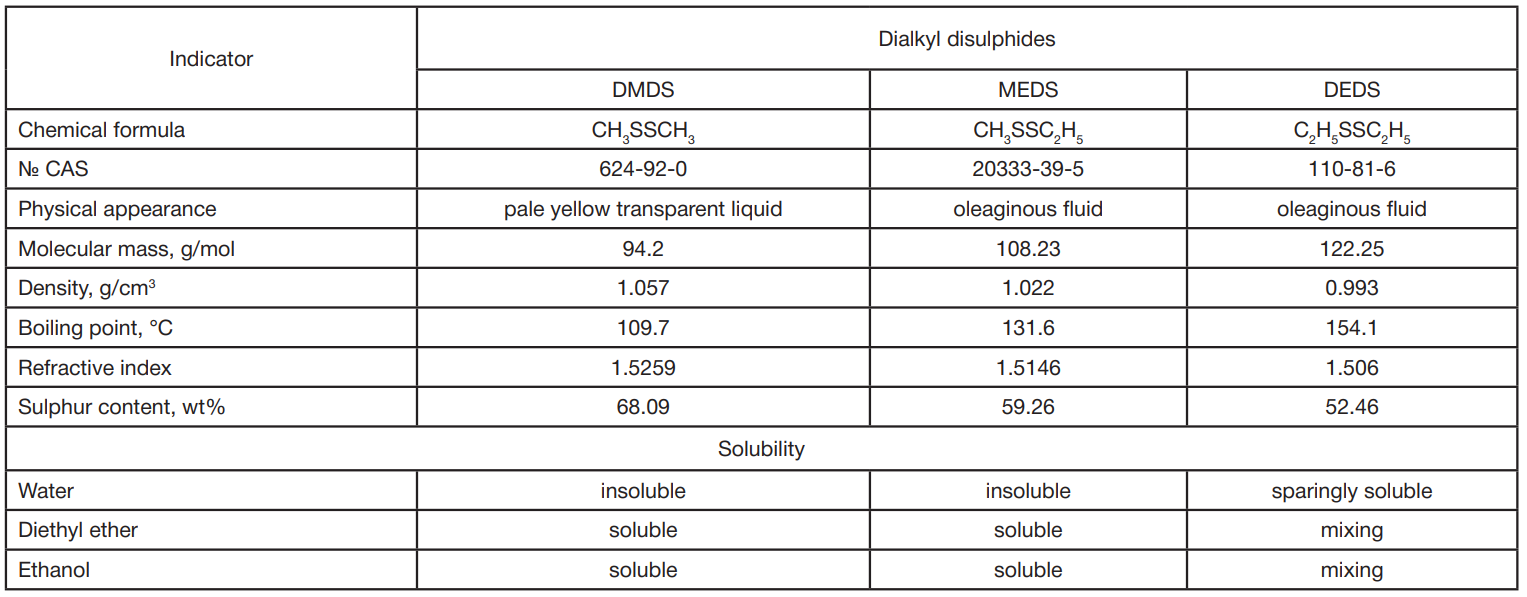
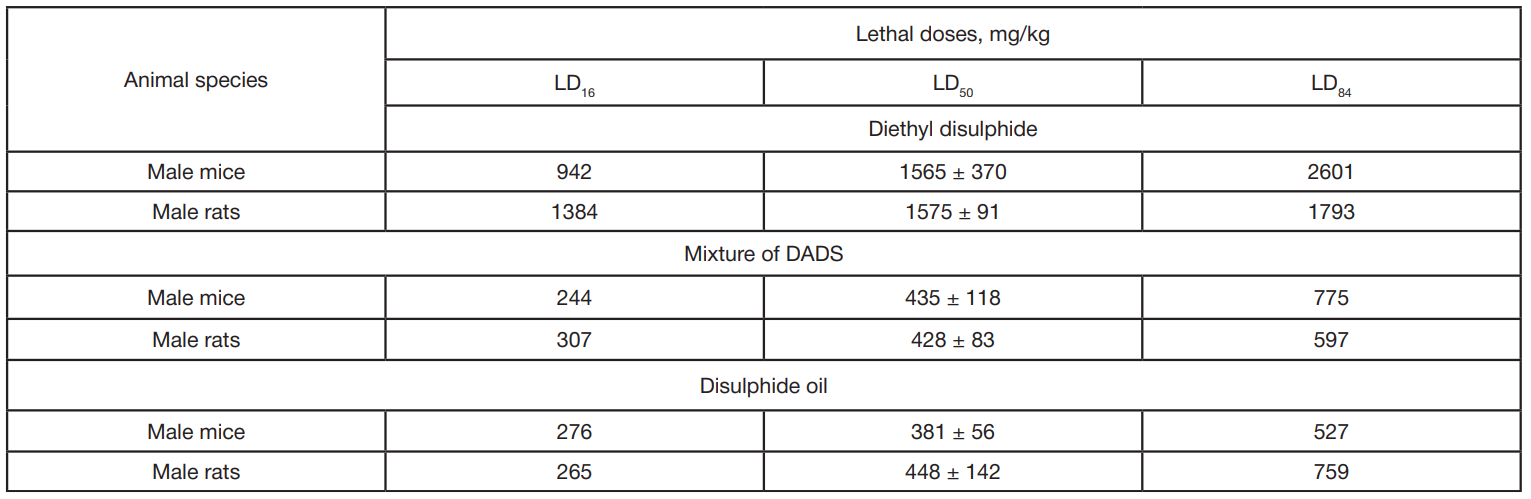
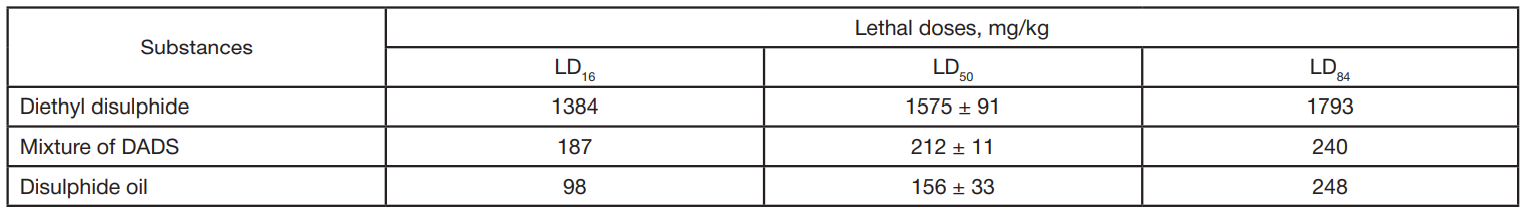
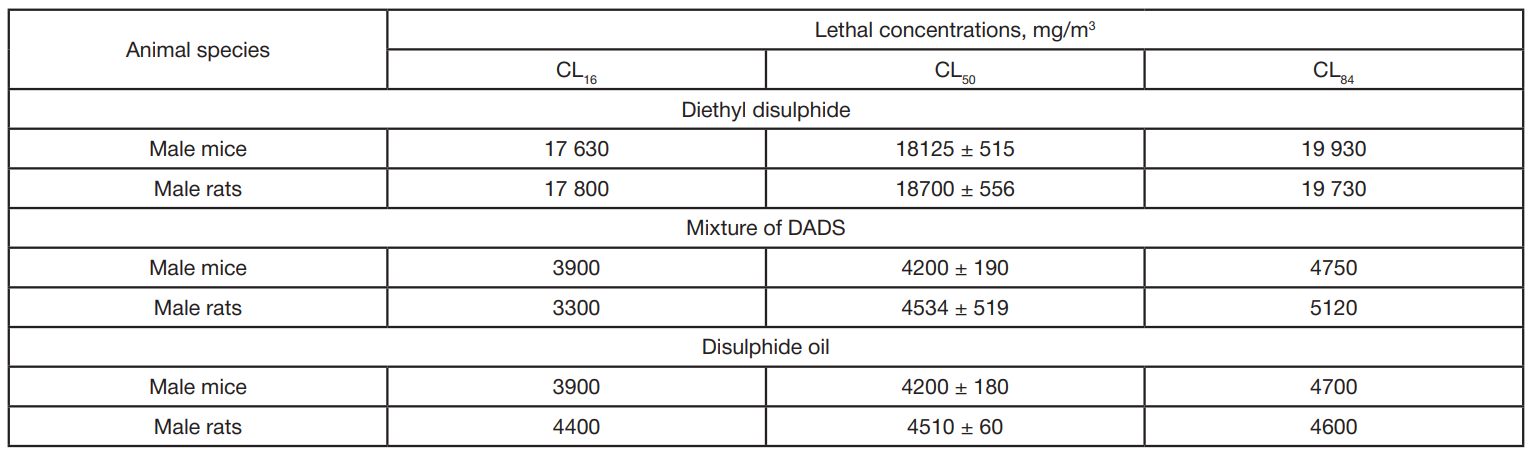
In vivo toxicity study of dialkyl disulphides
1 Research Institute of Hygiene, Occupational Pathology and Human Ecology of the Federal Medical Biological Agency, St. Petersburg, Russia
2 Mechnikov North-Western State Medical University, Saint Petersburg, Russia
Correspondence should be addressed: Semen A. Kucherskoy
Kapitolovo station, bld. 93, p/o Kuzmolovsky, Vsevolozhsky District, Leningrad reg., 188663; ur.hcepg@ioksrehcuk
Acknowlegements: we would like to thank Shkaeva IE, the leading researcher at the Research Institute of Hygiene, Occupational Pathology and Human Ecology of the FMBA, for study management, and Nikolaev AI, the leading researcher at the Research Institute of Hygiene, Occupational Pathology and Human Ecology of the FMBA, for assistance in mathematical processing of the results.
Author contribution: Kucherskoy SA – conducting toxicology studies.
Compliance with ethical standards: the study was approved by the Ethics Committee of the North-Western State Medical University named after I.I. Mechnikov (protocol № 8 dated November 11, 2020); laboratory animals were kept and fed in accordance with SP 2.2.1.3218-14 “Sanitary and Epidemiological Requirements for the Device, Equipment and Maintenance of Experimental Biological Clinics (Vivariums)”, as well as with the «Guide for Care and Use of Laboratory Animals» (USA).