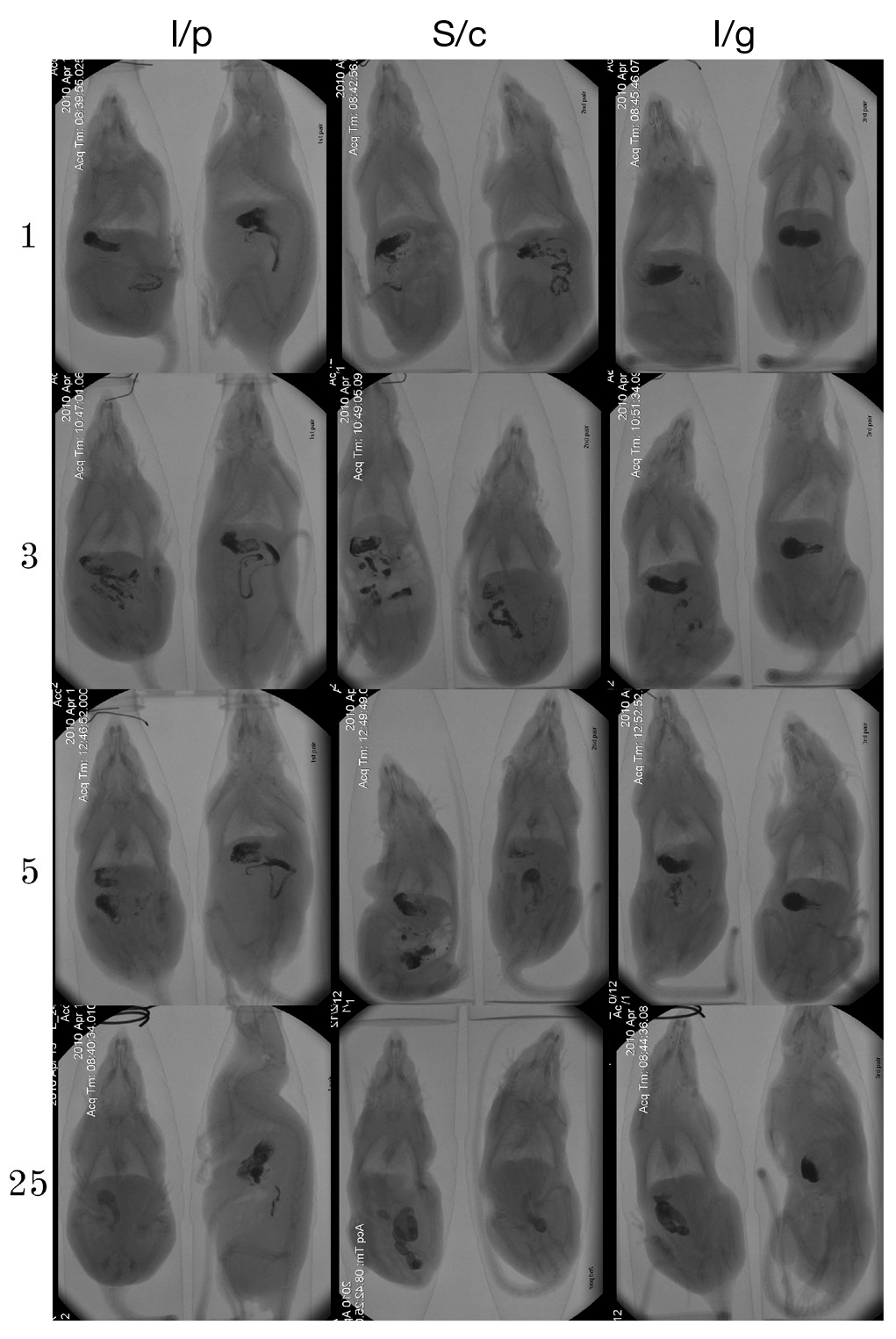
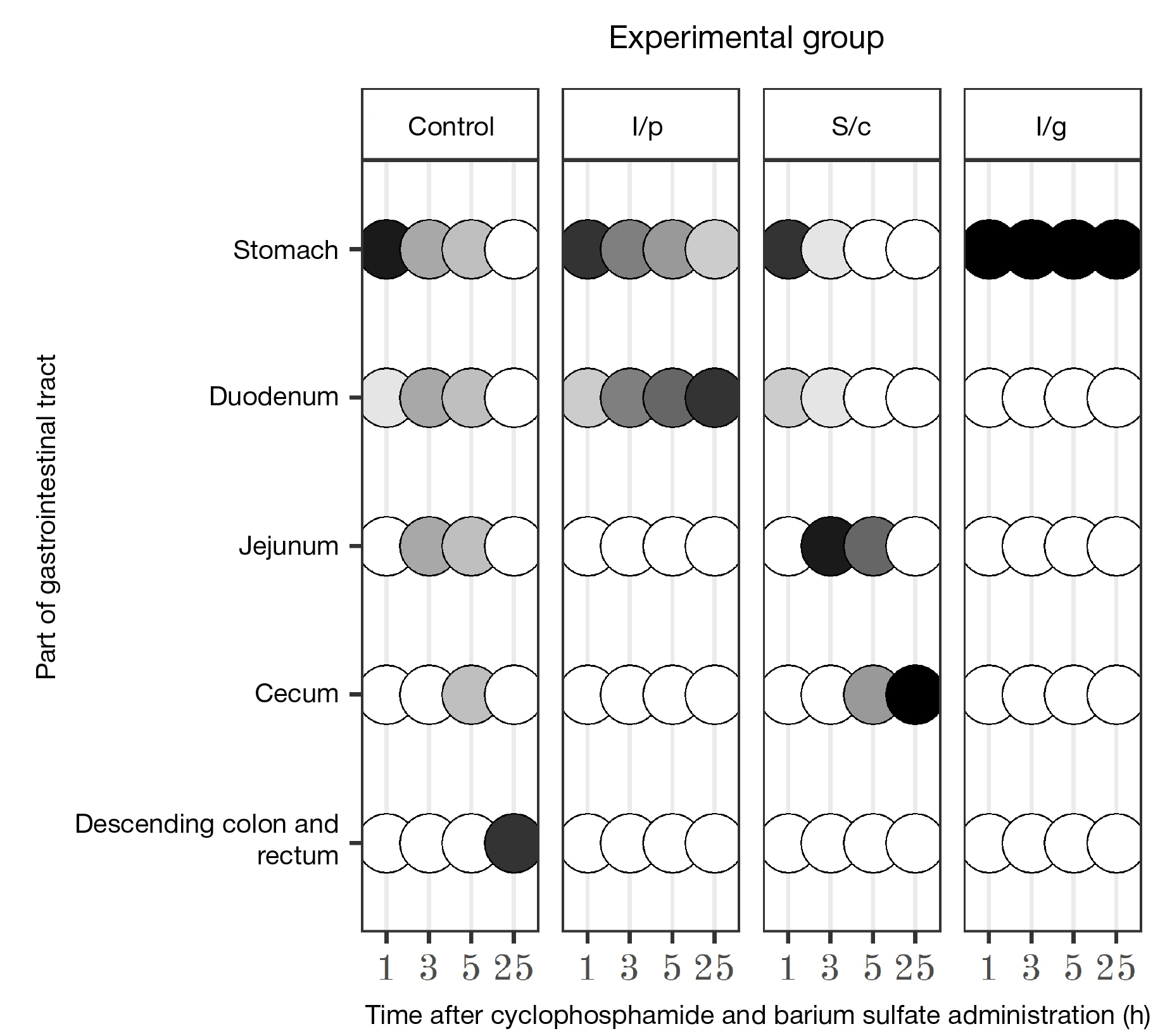
ORIGINAL RESEARCH
Modelling myeloablative cytostatic therapy with cyclophosphamide is accompanied by gastrointestinal stasis in rats
1 State Scientific Research Test Institute of the Military Medicine of Defense Ministry of the Russian Federation, Saint-Petersburg, Russia
2 Golikov Research Clinical Center of Toxicology of the Federal Medical Biological Agency, Saint-Petersburg, Russia
Correspondence should be addressed: Timur V. Schäfer
Lesoparkovaya, 4, Saint-Petersburg, 195043; ur.xednay@refahcs
Author contribution: Schäfer TV — developing the experimental model, study planning, experimental procedure, data processing and visualization; Ivnitsky JuJu — rationale, developing the experimental model, data interpretation; Rejniuk VL — setting up the experiment. All authors contributed to discussion, manuscript writing and editing.
Compliance with ethical standards: the study was carried out in accordance with the principles of bioethics, approved by the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (ETS N 123).