This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
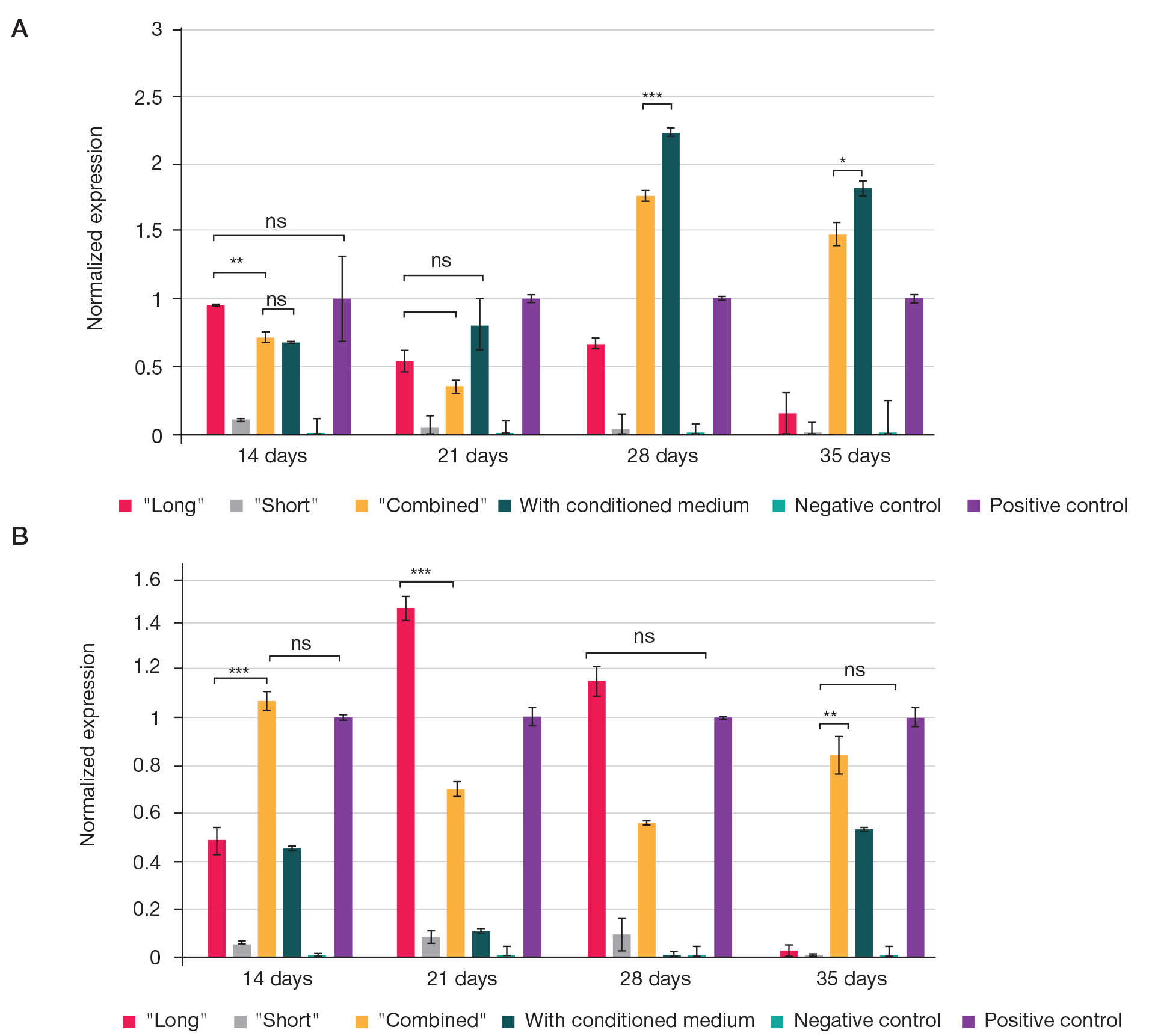
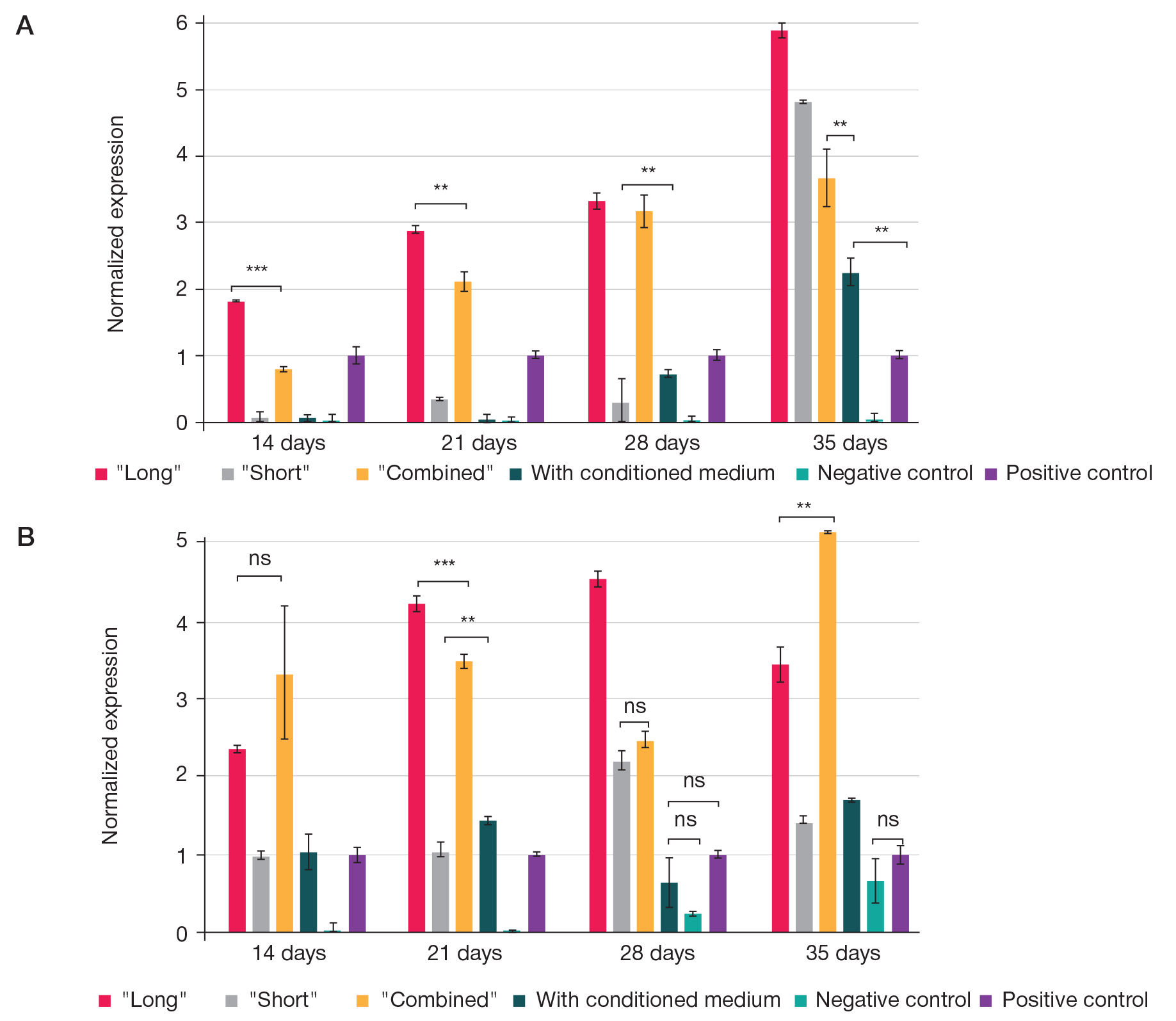
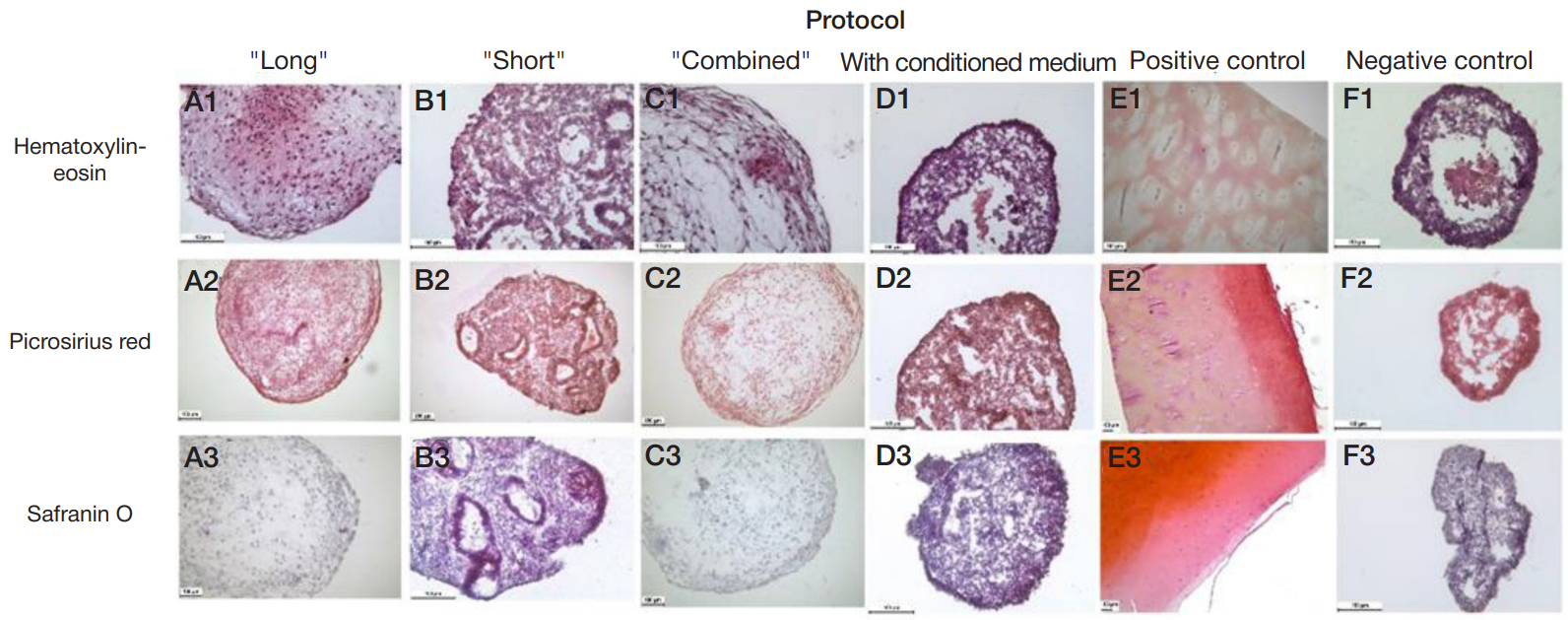
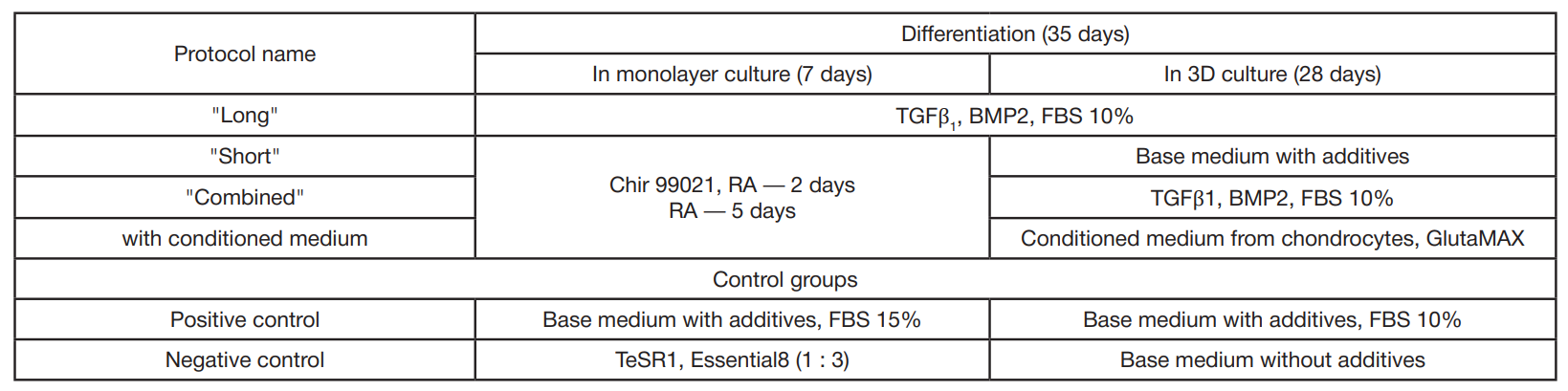
Fabrication of cartilage tissue substitutes from cells with induced pluripotency
1 Federal Research and Clinical Center of Physical-Chemical Medicine of Federal Medical Biological Agency, Moscow, Russia
2 Lomonosov Moscow State University, Moscow, Russia
3 Koltzov Institute of Developmental Biology, Moscow, Russia
Correspondence should be addressed: Artem V. Eremeev
Malaya Pirogovskaya, 1a, 119435, Moscow, Russia; ur.xednay@veemere-tra
Funding: the study was supported with an allocation #22-15-00250 by the Russian Science Foundation.
Acknowledgments: the authors thank Corresponding Member of the Russian Academy of Sciences M.A. Lagarkova for providing research facilities for the study
Author contribution: Eremeev AV — design of the experiment, general guidance, article authoring; Pikina AS — literature review, collection of the material, participation in the experimental part of the work, analysis of the resulting data; Ruchko ES — participation in the experimental part of the work; Sidorov VS, Ragozin AO — provision of material for the experiment.
Compliance with ethical standards: the study was performed in conformity with the principles of the Declaration of Helsinki (2000) and its subsequent revisions.