This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
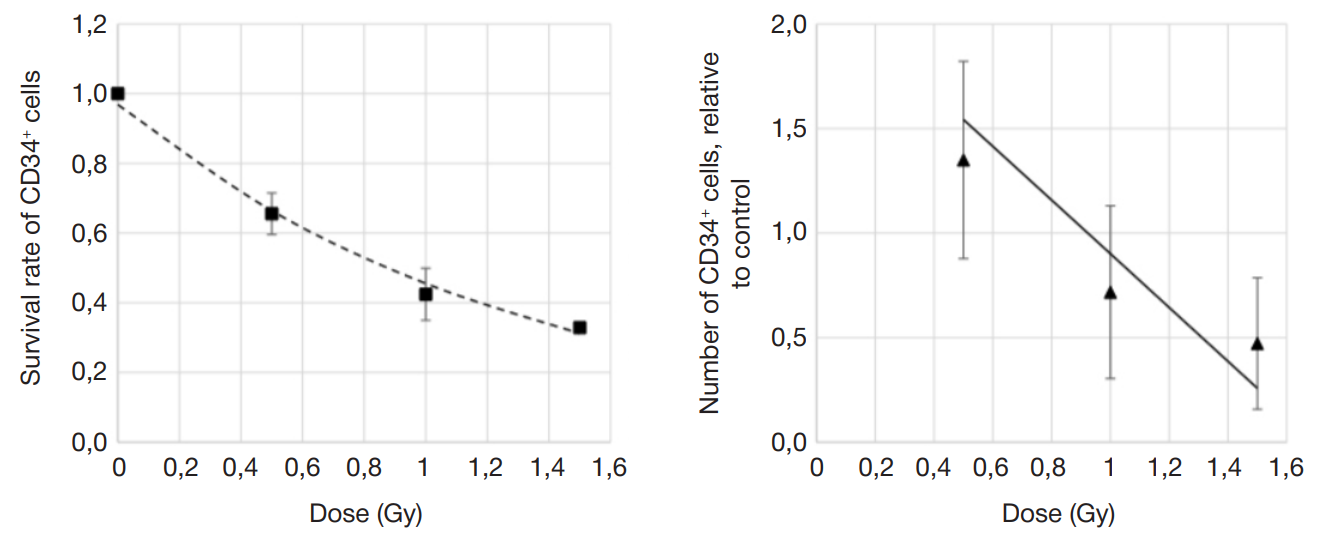
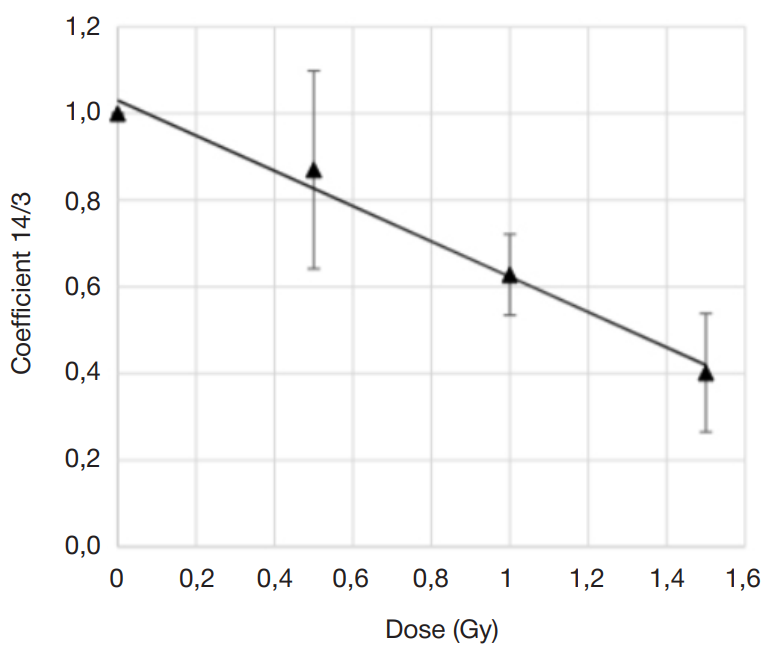
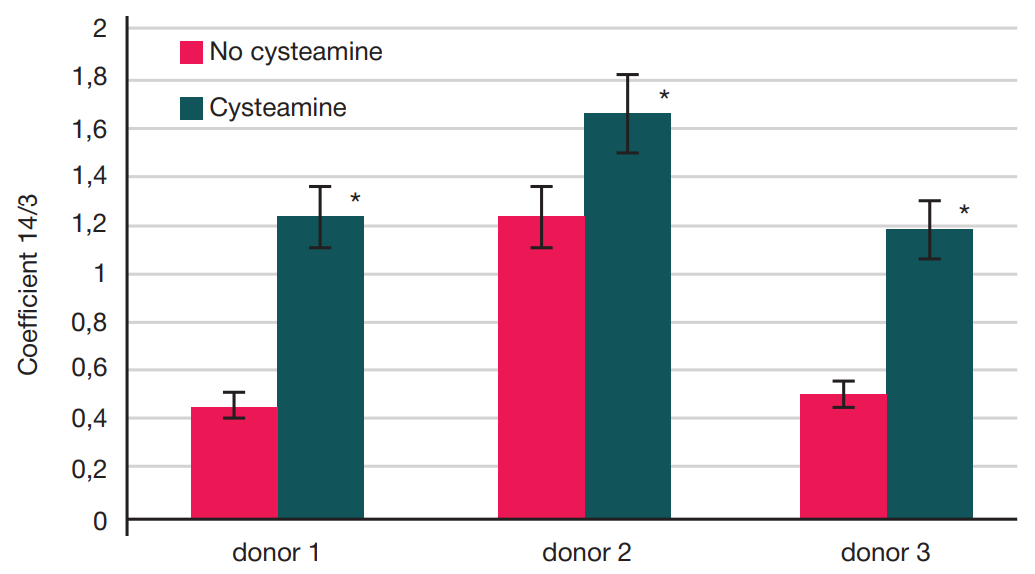
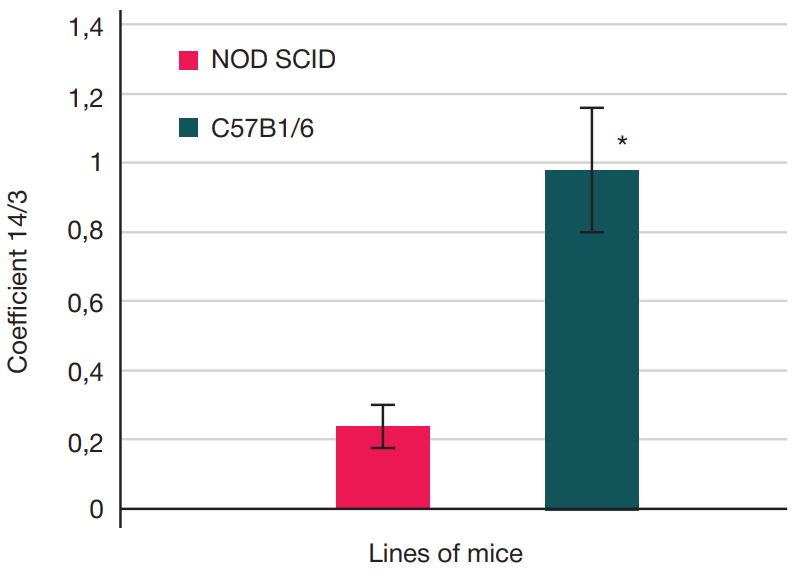
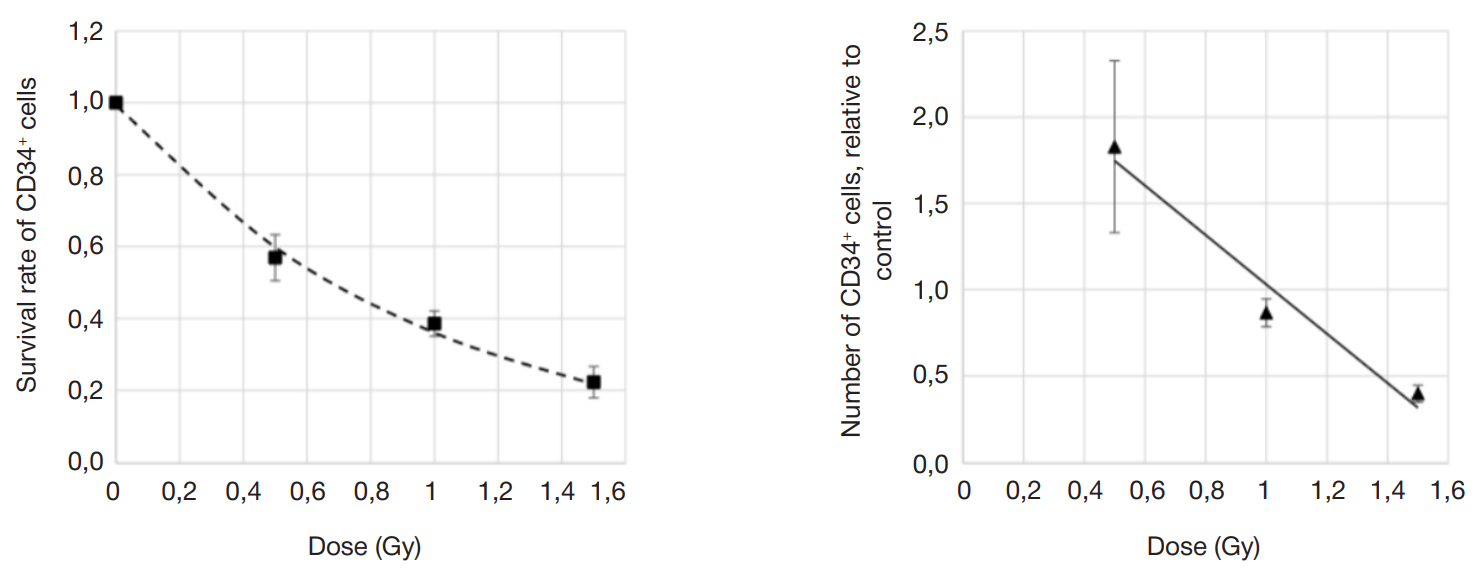
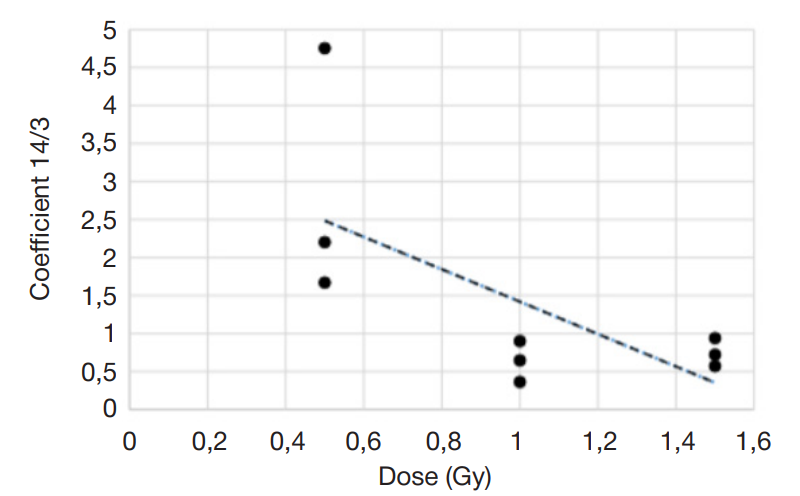
Assessment of individual hematopoietic stem cell response to gamma exposure using humanized mice
1 Urals Research Center for Radiation Medicine of the Federal Medical Biological Agency, Chelyabinsk, Russia
2 Chelyabinsk State University, Chelyabinsk, Russia
Correspondence should be addressed: Natalia I. Atamanyuk
Vorovskogo, 68А, Chelyabinsk, 454141, Russia; ur.liam@arhlup_ativ
Funding: the study was performed as part of the State Assignment of FMBA of Russia.
Acknowledgments: the authors would like to thank A.V. Sherstobitov (Regional Perinatal Center, Chelyabinsk) for his help in organizing and cord blood sampling.
Author contribution: Atamanyuk NI — planning and conducting experiments, manuscript writing; Pryakhin EA — study planning and management, manuscript writing; Styazhkina EV — HSC isolation, data analysis; Obvintseva NA — measurement, flow cytometry; Tryapitsyna GA — data analysis; Peretykin AA — animal exposure, dosimetry studies; Andreev SS — animal handling, measurement; Aldibekova AE — animal handling; Akleyev AV — general management.
Compliance with the ethical standards: the study was approved by the Ethics Committee of the Urals Research Center for Radiation Medicine (protocol № 2 of 27 June 2022). Cord blood sampling was performed after obtaining the informed consent from donors at the Chelyabinsk Regional Perinatal Center. Peripheral blood samples were provided by the Blood Transfusion Station of FMBA of Russia (Chelyabinsk) in accordance with the Decree of the Government of the Russian Federation № 331 of 12 April 2013. Animals were handled in accordance with the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (1986, Strasbourg), Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes.