This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
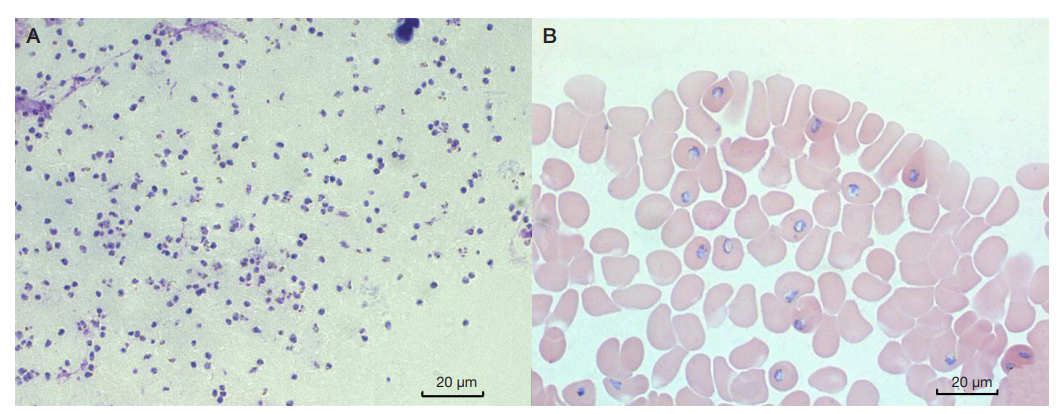
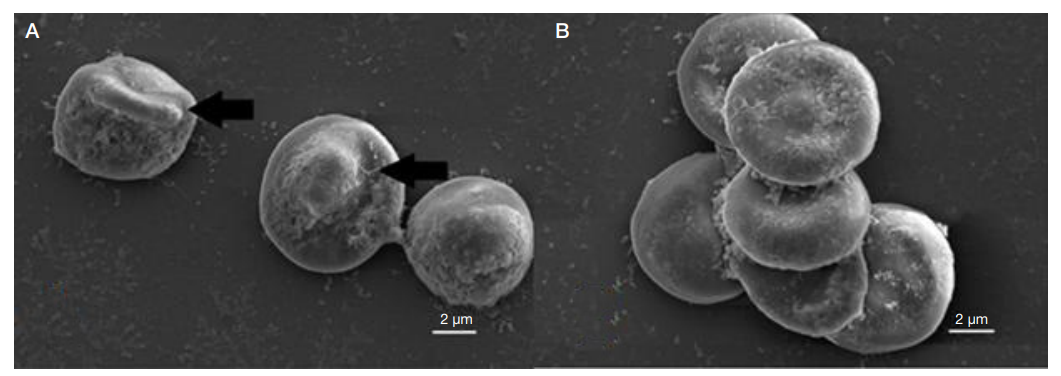
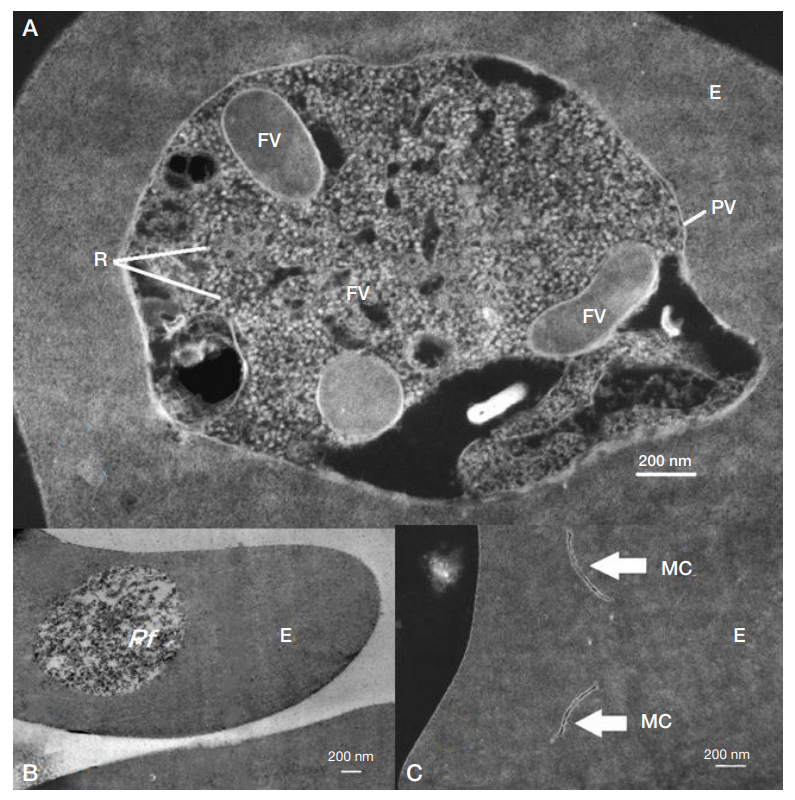
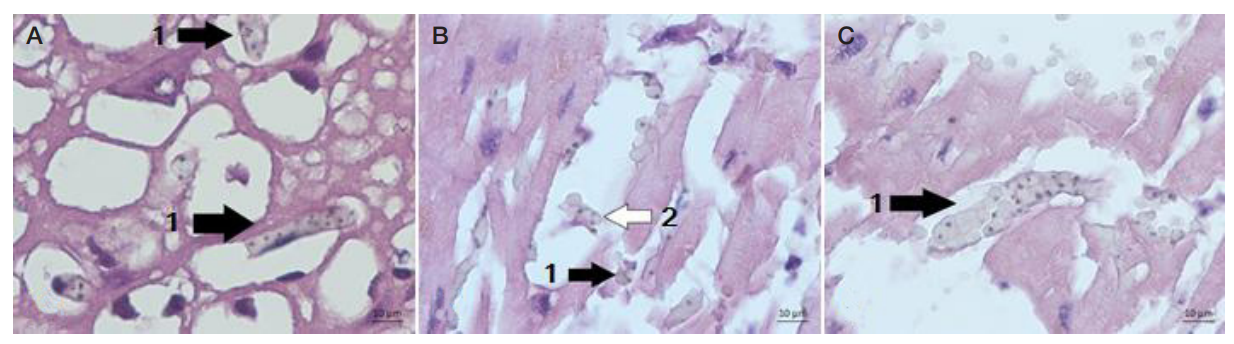
Electron microscopy of the Plasmodium falciparum trophozoites and the tissues these have infected in severe tropical malaria
1 Kirov Military Medical Academy, Saint-Petersburg, Russia
2 Botkin Clinical Infectious Diseases Hospital, Saint-Petersburg, Russia
3 Pediatric Research and Clinical Center of Infectious Diseases of the Federal Medical Biological Agency, Saint Petersburg, Russia
Correspondence should be addressed: Artem R. Ariukov
Akademika Lebedeva, 6, Saint-Petersburg, 194044, Russia; аur.xednay@metra.vokur
Author contribution: Solovev AI — concept, scientific justification, organization of all types of tests, analysis of the results, manuscript writing; Kapacina VA — data acquisition, practical advising; Sokolova MO, Ariukov AR — sample preparation, light microscopy, analysis of the results, manuscript writing; Kovalenko AN — practical justification, organization of data acquisition, manuscript editing; Uskov AN — concept, scientific advising; Romanenko VA — sample preparation, light microscopy, analysis of the results.
Compliance with ethical standards: the study was approved by the Ethics Committee of the Kirov Military Medical Academy (protocol No. 285 dated 21 November 2023) and conducted in accordance with the principleы of the Declaration of Helsinki (1964) and its subsequent updates.