This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
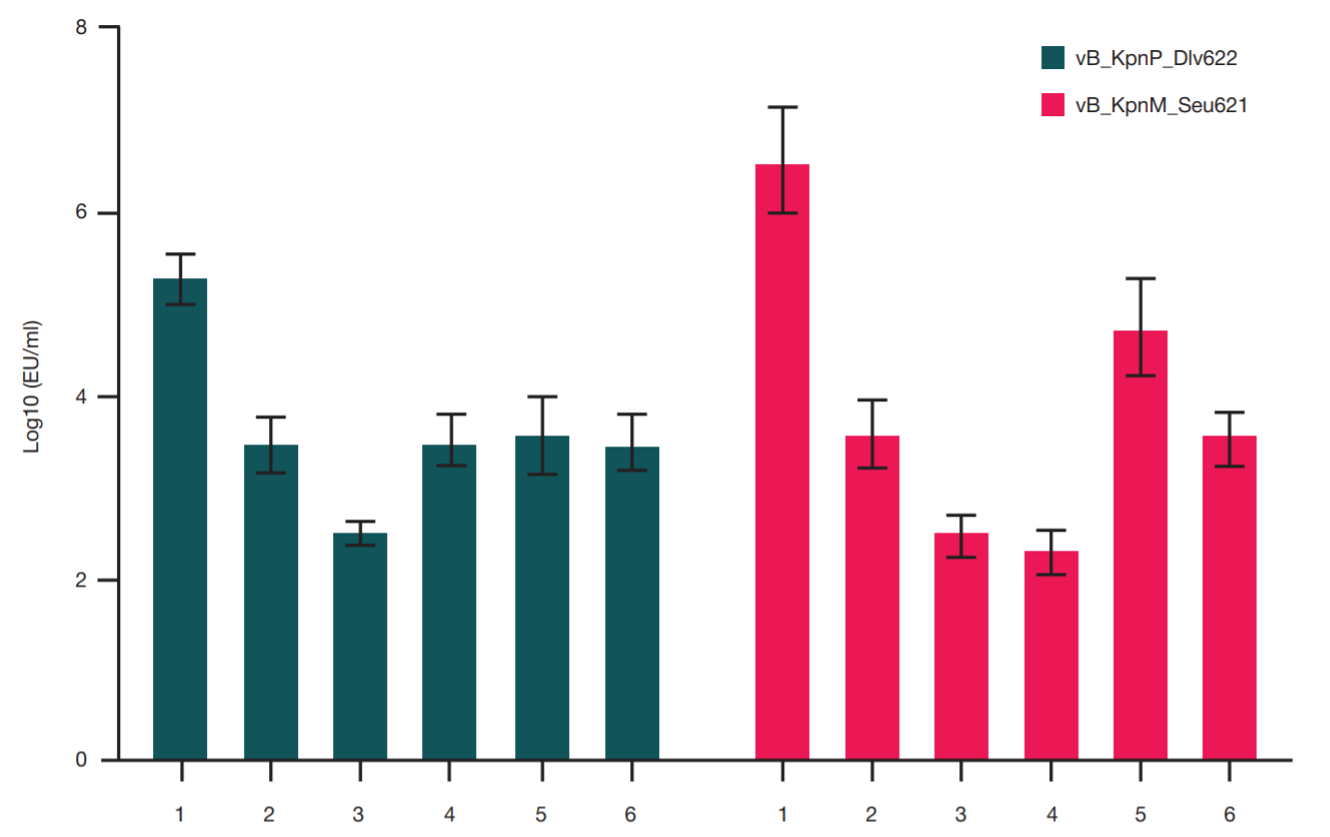
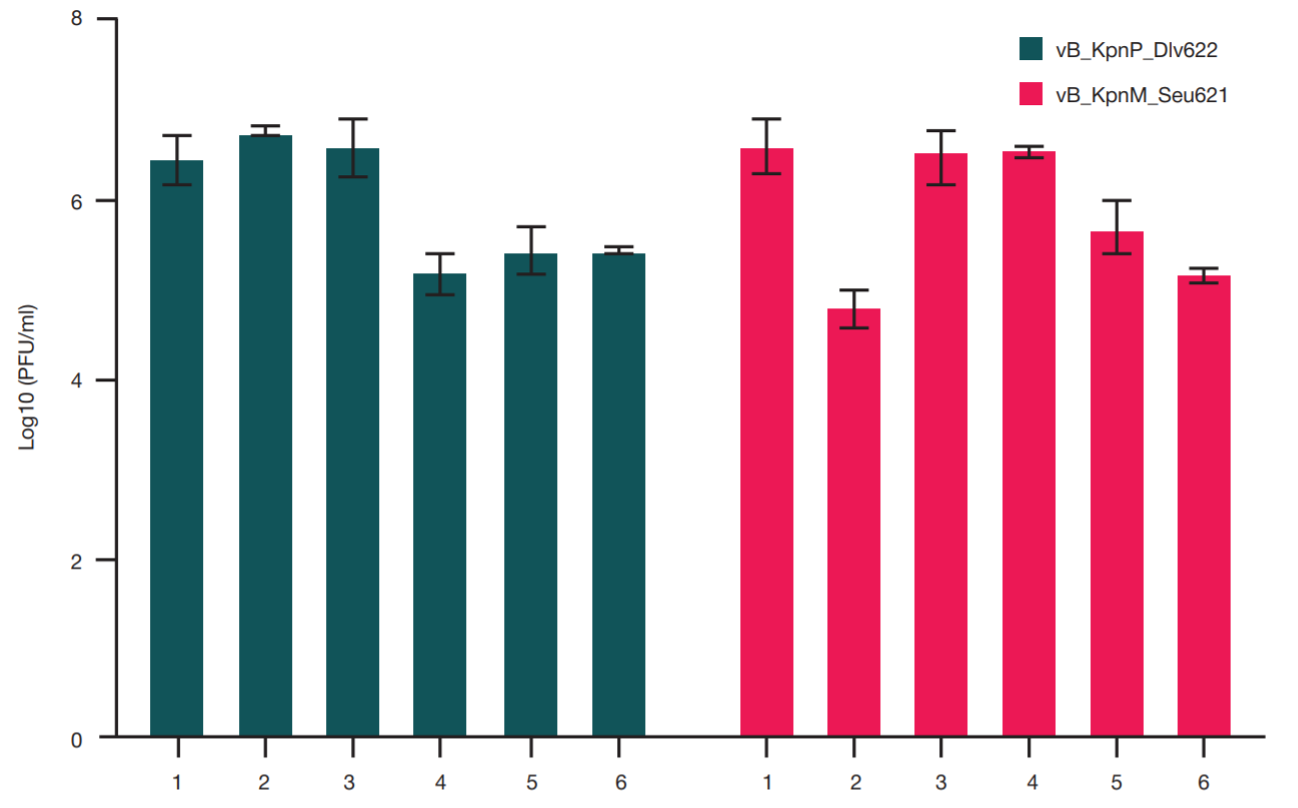
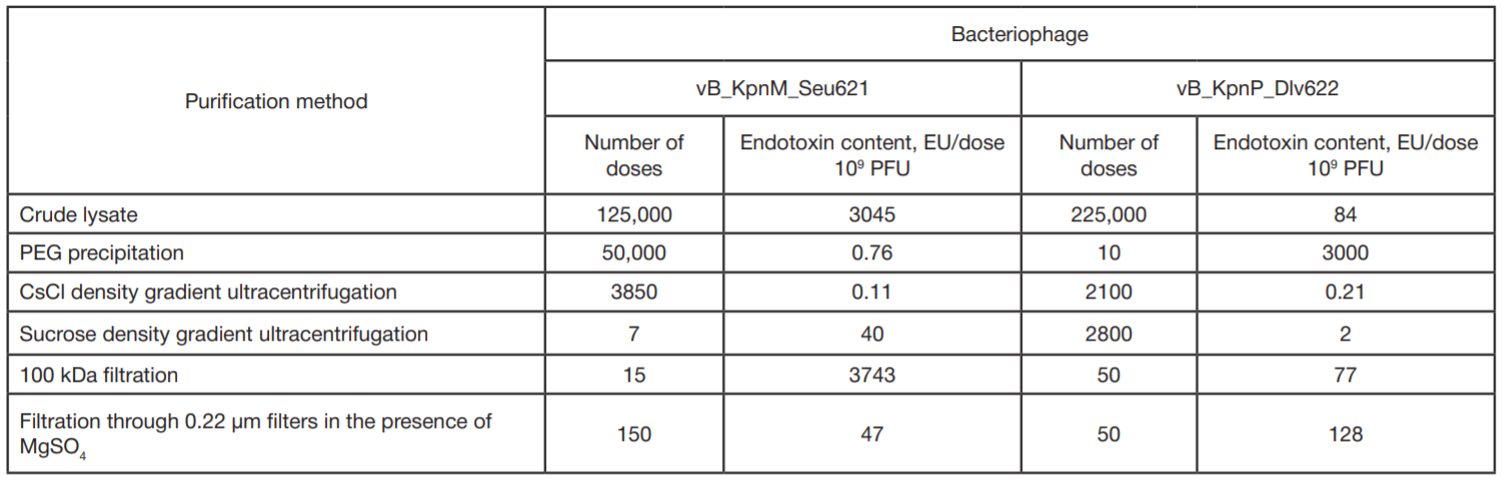
Comparison of methods for purification of bacteriophage lysates of gram-negative bacteria for personalized therapy
1 Federal Research and Clinical Center of Physical-Chemical Medicine of Federal Medical Biological Agency, Moscow, Russia
2 Federal Research Center of Biotechnology, Moscow, Russia
Correspondence should be addressed: Roman B. Gorodnichev
Malaya Pirogovskaya, 1a, Moscow, 119435; moc.liamg@b.r.vehcindorog
Funding: all study expenses were covered by the funds allocated for the State Assignment on the Development of a personalized approach to the therapy of infections using virulent bacteriophages (Code: Bacteriophage).
Author contribution: Gorodnichev RB — planned the study, conducted the experiments, and wrote the manuscript; Kornienko MA, Letarov AV, Shitikov EA — planned the study, analyzed its results, and wrote the manuscript; Kuptsov NS, Efimov AD, Bogdan VI — conducted the experiments; Ilina EN — planned the study and wrote the manuscript.
Compliance with ethical standards: the experiments were conducted in full compliance with Biosafety Guidelines for working with risk group III–IV pathogens (SP 1.3.2322-08), Amendment 1 to Biosafety Guidelines for working with risk group III–IV pathogens (SP 1.3.2518-09), medical waste regulations (SanPin 2.1.7.279010), and Federal Clinical Guidelines on the rational use of bacteriophages for therapy and prevention of diseases.