This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
METHOD
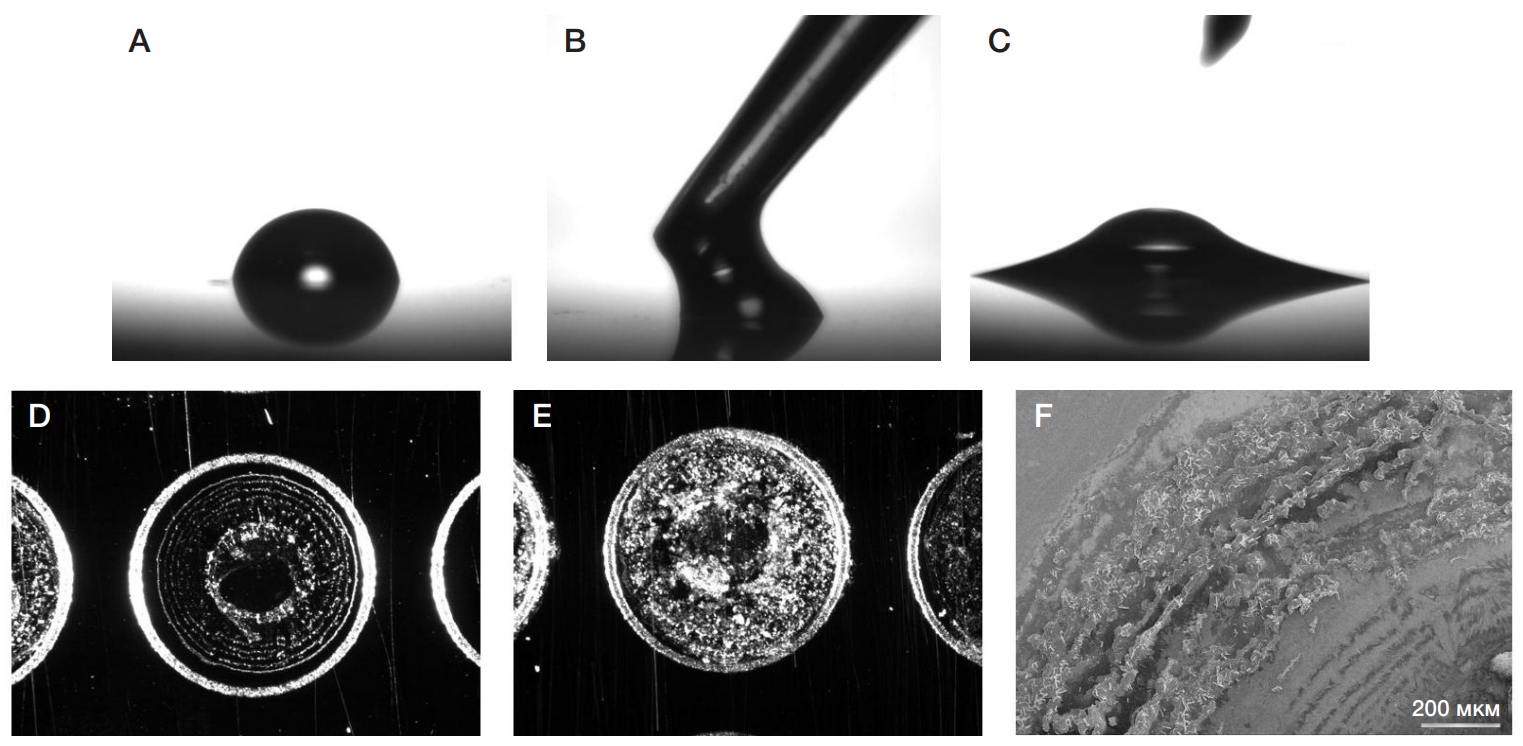
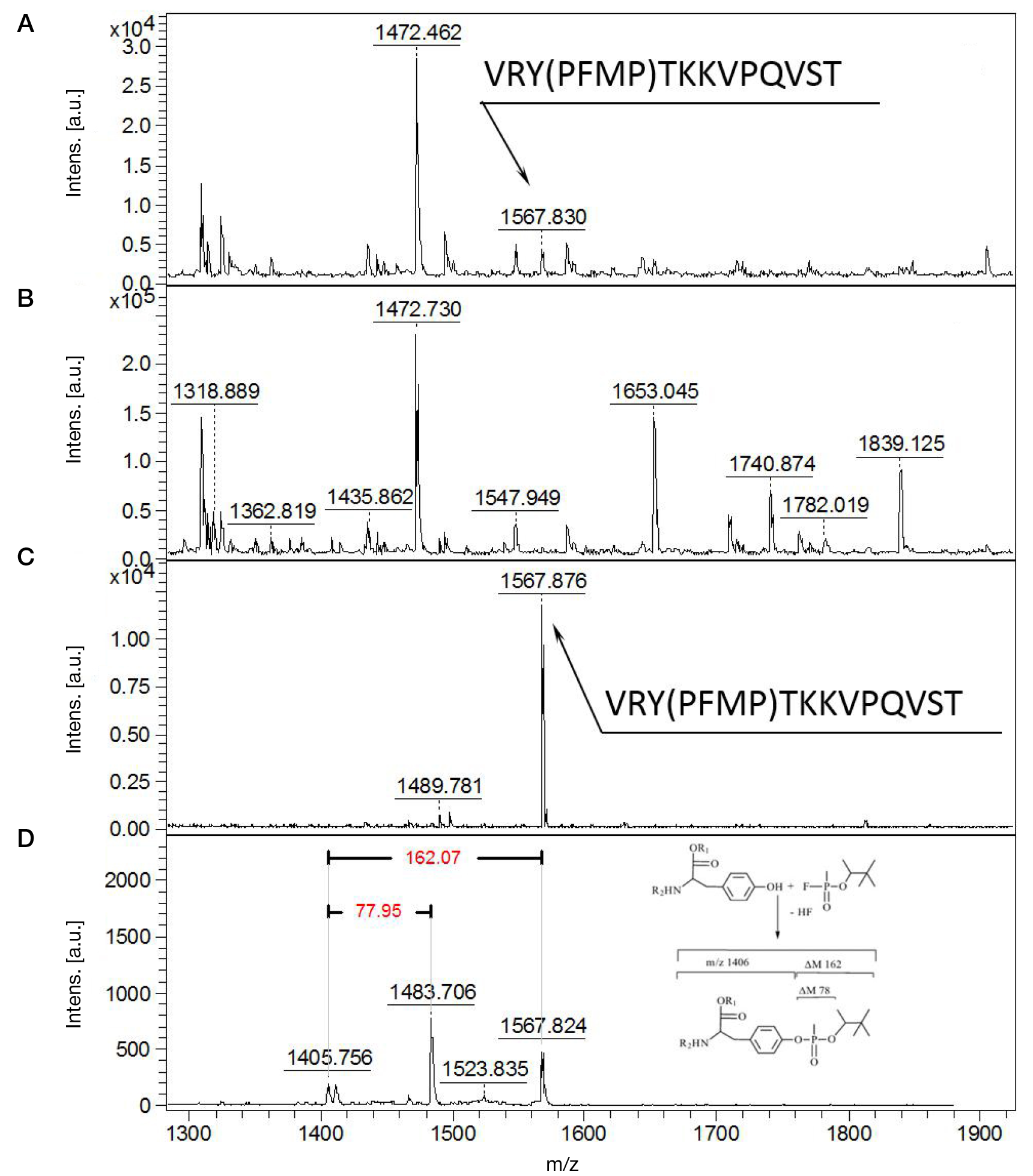
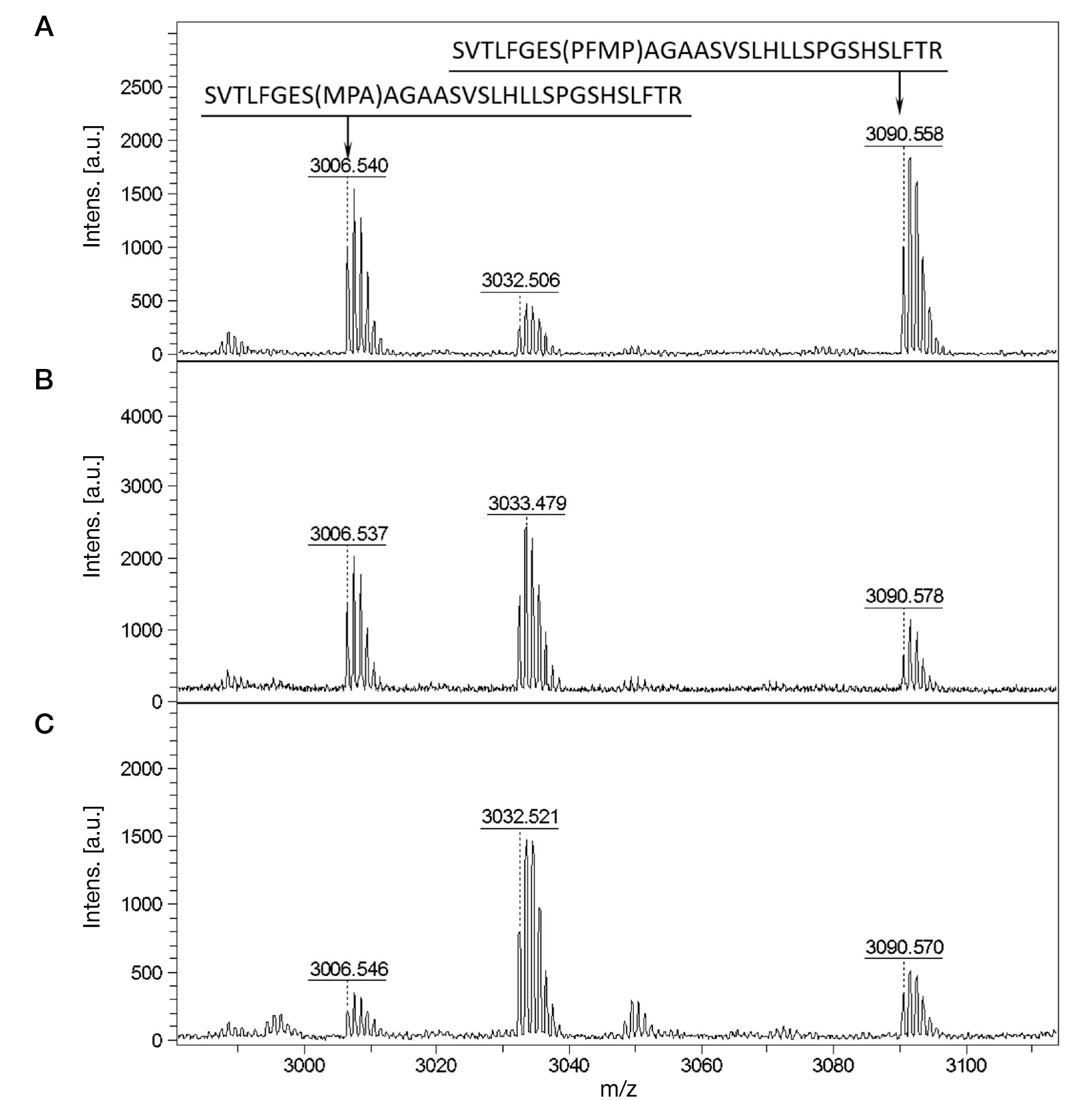
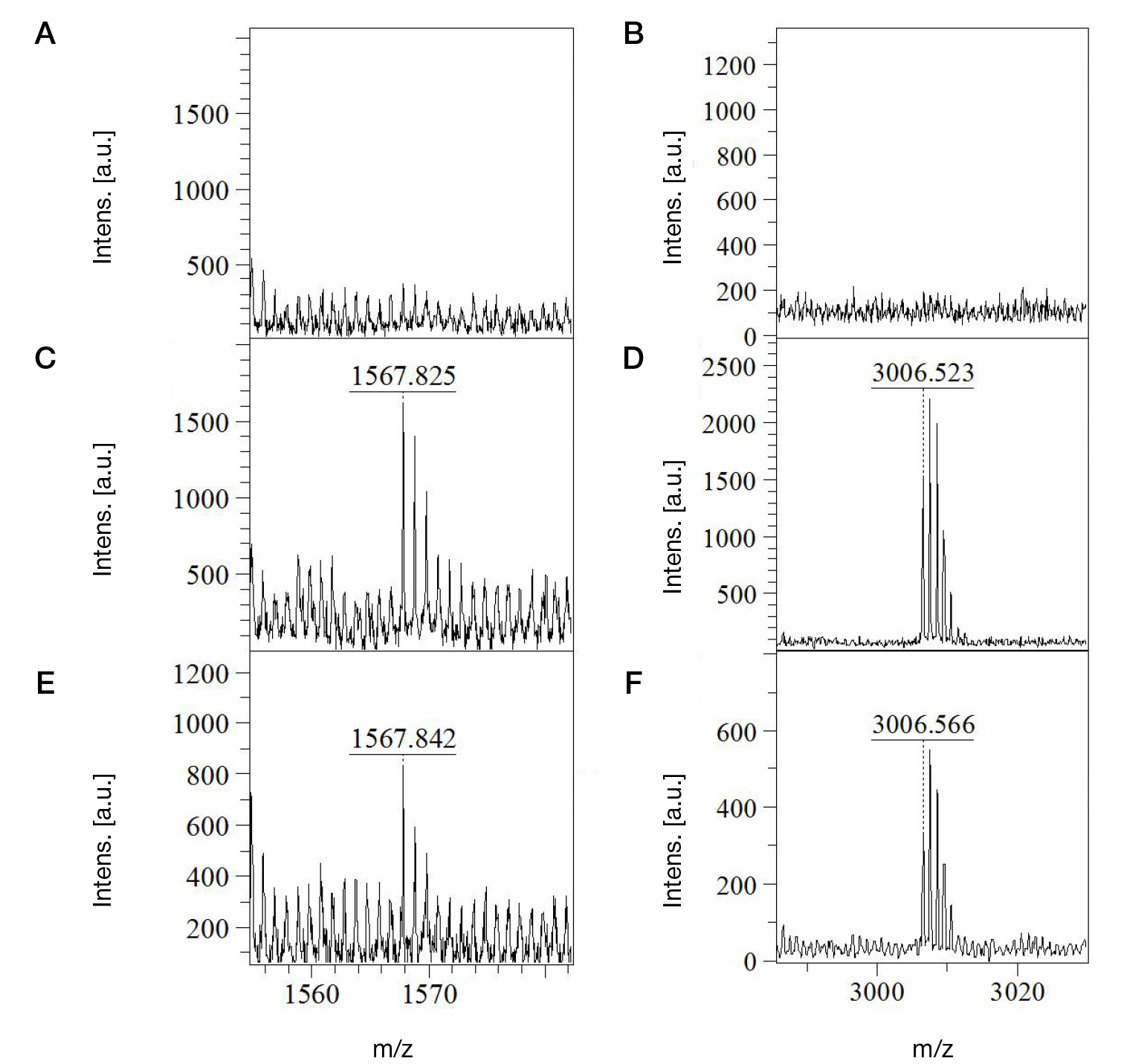
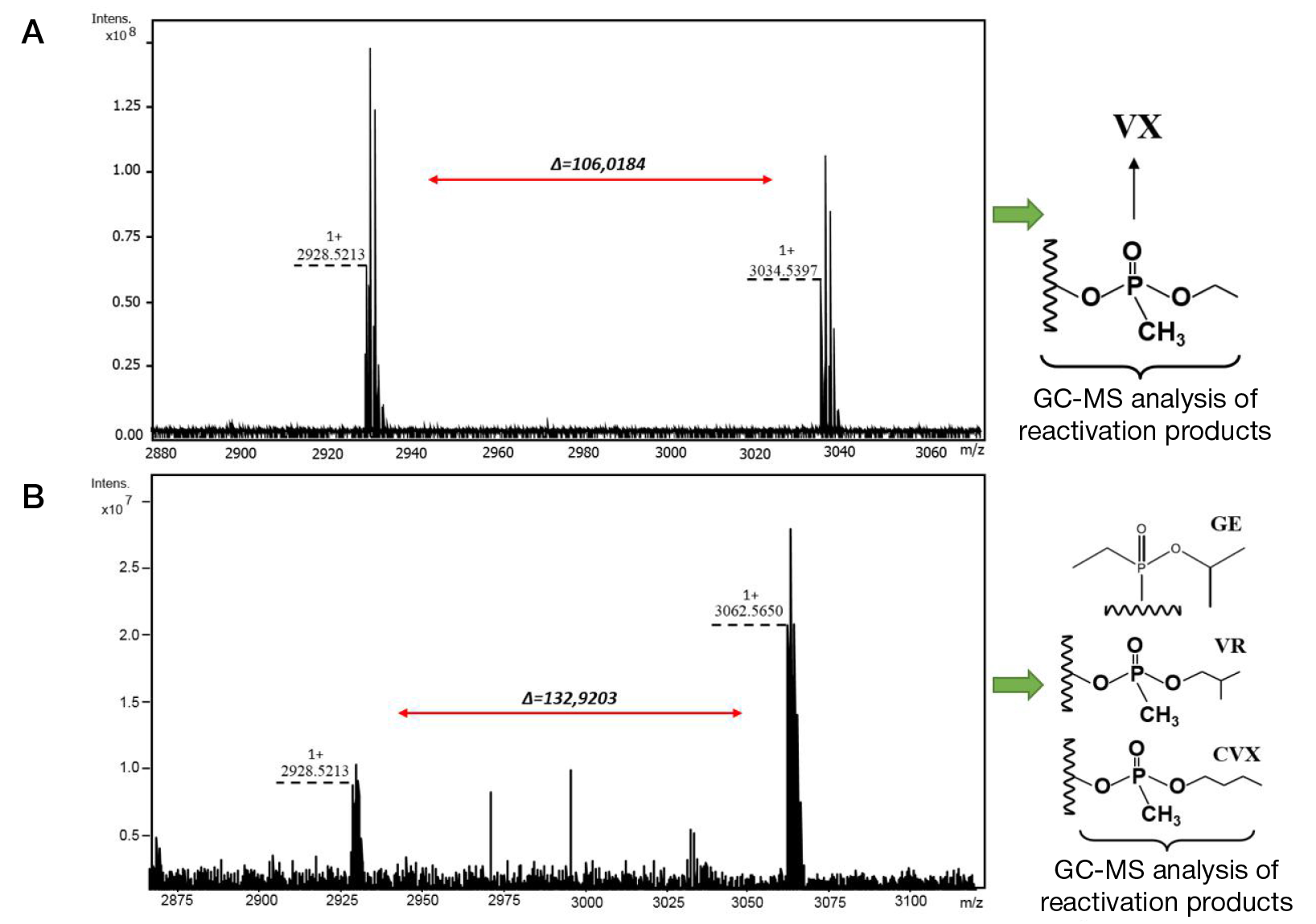
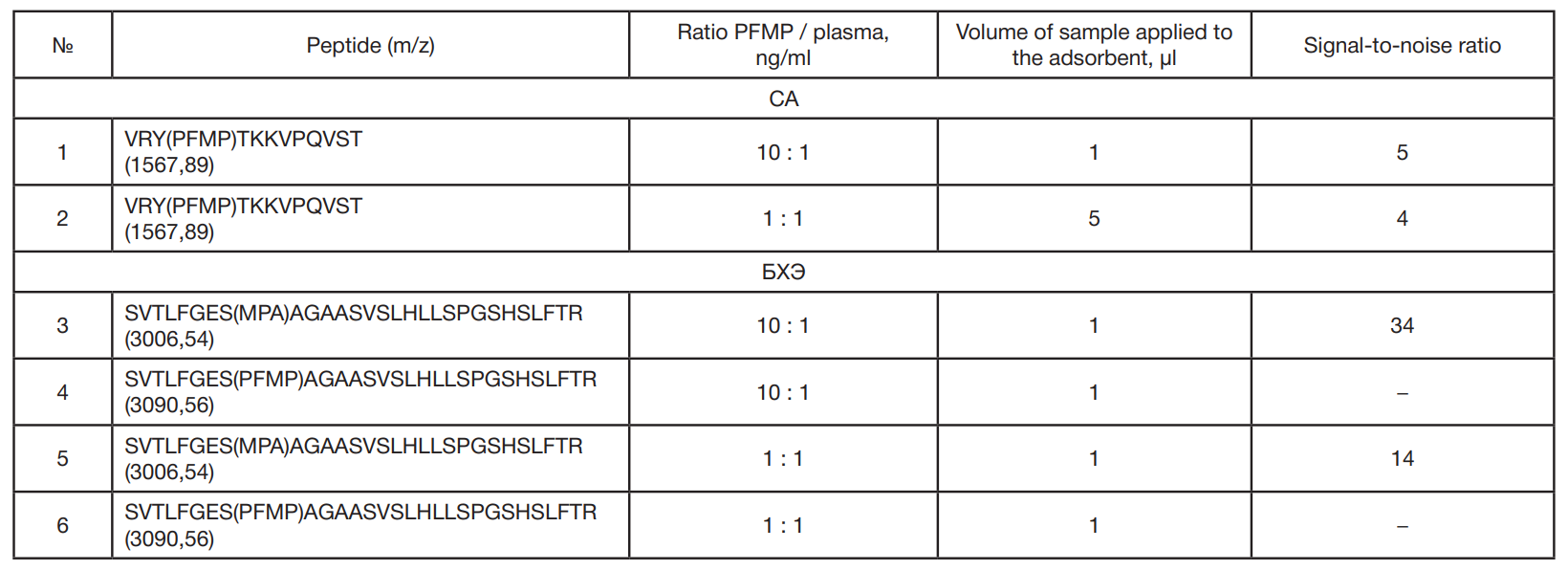
Identification of phosphonylated peptides using a MALDI target functionalized with lanthanum stearate
1 Research Institute of Hygiene, Occupational Pathology and Human Ecology of the Federal Medical Biological Agency, St. Petersburg, Russia
2 Golikov Research Center of Toxicology, St. Petersburg, Russia
3 Institute for Analytical Instrumentation, Russian Academy of Sciences, St. Petersburg, Russia
4 St. Petersburg State University, St. Petersburg, Russia
Correspondence should be addressed: Vladimir Nikolaevich Babakov
Kuzmolovsky, st. Kapitolovo, str. 93, Leningradsraja oblast, Russia; ur.hcepg@vokabab
Acknowledgments: the authors express their gratitude for technical support to the resource centers Development of Molecular and Cellular Technologies and Geomodel of the Research Park of St. Petersburg State University, and to A.A. Selyutin for the opportunity to use the MALDI mass spectrometer.